
South Korea in Vitro Diagnostics Market Size, Share, Trends and Forecast by Test Type, Product, Application, End User, and Region, 2026-2034
South Korea in Vitro Diagnostics Market Size Overview:
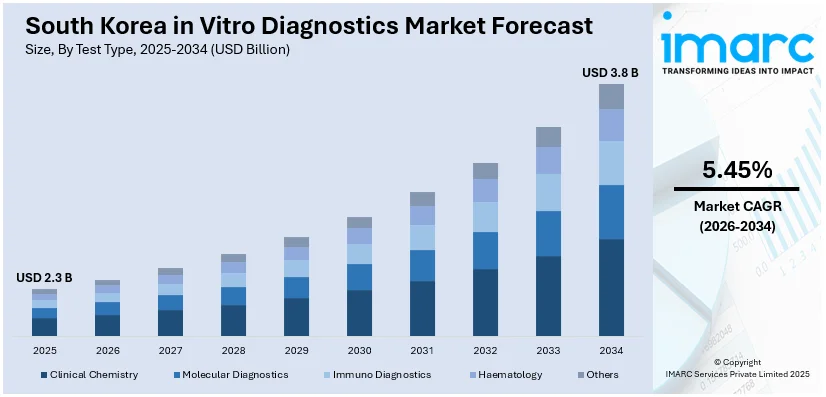
The South Korea in vitro diagnostics market size was valued at USD 2.3 Billion in 2025. Looking forward, IMARC Group estimates the market to reach USD 3.8 Billion by 2034, exhibiting a CAGR of 5.45% from 2026-2034. The market in the region is market is driven by rapidly aging population, high prevalence of chronic diseases, ongoing technological advancements, strong government initiatives, and rising healthcare expenditure.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2025 |
|
Forecast Years
|
2026-2034
|
|
Historical Years
|
2020-2025
|
| Market Size in 2025 | USD 2.3 Billion |
| Market Forecast in 2034 | USD 3.8 Billion |
| Market Growth Rate (2026-2034) | 5.45% |
The South Korea in vitro diagnostics (IVD) market growth is primarily driven by its aging population, leading to a higher demand for health monitoring. With the growing elderly population, the demand for regular diagnostic testing to address age-related health concerns is increasing, driving market expansion. Additionally, the rising incidence of chronic conditions like diabetes, cardiovascular diseases, and cancer is boosting the need for diagnostic solutions. Moreover, ongoing technological advancements, including the development of point-of-care testing and personalized medicine, enhance diagnostic efficiency, increasing adoption and providing an impetus to the market. Also, government initiatives focused on enhancing healthcare infrastructure and regulatory support, contributing to the market expansion. For instance, the largest deployment of cholera rapid diagnostic tests sent 1.2 million units to 14 countries during 2024 with Malawi receiving the initial shipment. This highlights the significant role of South Korea in advancing global healthcare by providing timely diagnostic solutions in response to public health crises. Furthermore, rising healthcare expenditure in South Korea promotes the adoption of advanced diagnostic technologies, impelling the market growth.
To get more information on this market Request Sample
Concurrently, a significant driver in the surging South Korea IVD market demand is the increasing focus on precision medicine, with diagnostics playing a crucial role in tailoring treatments to individual needs. In line with this, the ongoing integration of artificial intelligence (AI) and machine learning (ML) into diagnostic processes enhances decision-making accuracy, encouraging healthcare providers to adopt these innovations, and fostering the market growth. Besides this, the rise of health-conscious consumers, seeking early detection and personalized health management is supporting the market demand. Also, strong collaborations between IVD companies and healthcare providers are enhancing testing capabilities, bolstering the market growth. For example, the initial official Gavi Vaccine Alliance distribution of tests will enable faster outbreak detection and monitoring as well as more effective surveillance of vaccination programs and enhanced targeting of disease prevention measures. Apart from this, a growing focus on preventive healthcare drives a shift towards early diagnosis, increasing the need for reliable IVD solutions, thereby propelling the market forward.
South Korea In Vitro Diagnostics Market Trends:
Technological Advancements in IVD
The South Korea IVD market trends experience major transformations because of ongoing technological advancements in in vitro diagnostics. The advancement of point-of-care testing and next-generation sequencing and molecular diagnostics has produced better and faster testing solutions in South Korea. For example, the digital health sector of South Korea served as an ideal destination for international companies to invest due to its advanced technological developments. The K-Hospital+Health Tech Fair 2023 presented visitors with new developments in South Korean healthcare technology. The market demand for advanced diagnostic tools also grows because these technological solutions enable early detection of cancer and infectious diseases. AI together with ML operates in diagnostic processes to maximize accuracy levels and streamlines workflows and develops personalized medical treatments. Furthermore, research predicts an upward trajectory of these diagnostic tools since technological innovations promise better healthcare results at more affordable costs, thus enhancing the South Korea IDV market outlook.
Shift Toward Preventive Healthcare
The South Korean healthcare system has changed direction toward disease prevention through early disease identification before conditions advance. Healthcare service providers now focus on decreasing chronic disease load which has led consumers to show higher engagement in their own health management. Moreover, the South Korean population demonstrates enhanced interest in scheduled medical examinations and individual health surveillance and diagnostic testing. For example, in 2023 the South Korean government invested ₩500 billion equivalent to USD 400 million for the advancement of diagnostic tools to expand early disease detection capabilities. The market for IVD solutions including blood tests and molecular diagnostics and home testing kits also expands because more people choose preventive healthcare. Apart from this, early diagnosis benefits continue to gain recognition which drives this market trend.
Expansion of Point-of-Care Diagnostics
As per the South Korea in vitro diagnostics market forecast, the country is experiencing increasing adoption of point-of-care diagnostics which transforms the market through rapid on-site testing solutions. The market is shifting in this direction because patients need more convenient access to tests which provide faster results from places other than traditional healthcare locations. Rapid infectious disease tests serve as point-of-care diagnostics which clinicians use in hospitals alongside clinics and patients perform at home. For instance, Seegene Inc. partnered with Microsoft, as its technology partner to boost POC diagnostic capabilities through its SG OneSystem business. The joint venture demonstrates how technological alliances help drive speedy innovation within the point-of-care diagnostic industry. Also, the existing healthcare infrastructure expansion together with cost-effective solution demands strengthens the market acceptance of POC testing. As a result, South Korea advances its healthcare accessibility agenda through these changes which lower healthcare expenses while maintaining fast dependable results, providing an impetus to the market.
South Korea In Vitro Diagnostics Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the South Korea in vitro diagnostics market, along with forecasts at the country and regional levels from 2026-2034. The market has been categorized based on test type, product, application, and end user.
Analysis by Test Type:
- Clinical Chemistry
- Molecular Diagnostics
- Immuno Diagnostics
- Haematology
- Others
Clinical chemistry tests are essential for disease diagnosis, as they analyze blood, urine, and other bodily fluids to provide critical health insights. These tests help in assessing organ function, detecting metabolic disorders, and monitoring chronic conditions like diabetes, driving growth in the IVD market.
Medical testing through molecular diagnostics detects genetic substances DNA and RNA to identify diseases, genetic conditions and cancer types. The sector is expanding quickly because technological developments allow precise diagnosis while enabling earlier detection of diseases and customized therapeutic approaches.
The diagnostic technique of immuno diagnostics uses antibodies together with antigens to detect diseases which helps identify infections and allergies and autoimmune disorders. Additionally, immunoassays continue to gain popularity because they deliver fast accurate testing solutions across multiple disease categories.
Hematology tests examine blood components to diagnose conditions such as anemia, leukemia, and clotting disorders. Growing demand for blood diagnostics, coupled with technological advancements and increasing blood disorder cases, is driving this segment's growth.
Analysis by Product:
- Instrument
- Reagent
- Others
The instrument segment in the South Korea IVD market is crucial for performing diagnostic tests. The IVD market features devices such as analyzers and diagnostic machines and laboratory equipment which provide both precision and reliability. The combination of continuous technological improvements including automation and miniaturization has boosted the need for innovative instruments which supports both efficiency and cost-effectiveness in medical facilities including hospitals and research laboratories, strengthening the South Korea IDV market share.
Reagent is essential for diagnostic tests as they enable laboratory professionals to detect specific biomarkers and pathogens and genetic material. The regions’ reagent market continues to grow because of rising needs for personalized medicine and advanced diagnostic capabilities. Reagents of high quality enable accurate patient care through molecular diagnostics and clinical chemistry while improving both reliability and efficiency, fueling the market demand.
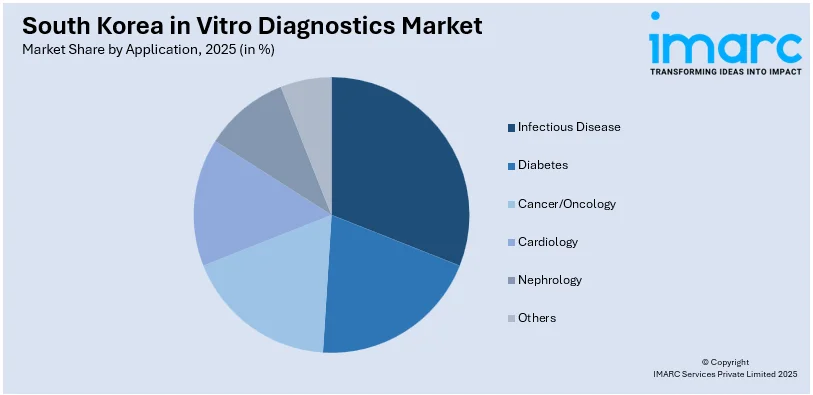
Analysis by Application:
Access the comprehensive market breakdown Request Sample
- Infectious Disease
- Diabetes
- Cancer/Oncology
- Cardiology
- Nephrology
- Others
Infectious disease diagnostics work to identify pathogens such as bacteria, viruses and fungi by using molecular tests together with cultures and immunoassays. The high number of infectious diseases across populations creates an urgent requirement for swift accurate testing solutions that support early diagnosis and medical intervention, boosting the market growth.
Diabetes diagnostics, including blood glucose testing and HbA1c monitoring, are critical for managing diabetes in South Korea. The increasing prevalence of diabetes is driving demand for advanced diagnostic tools that enable early detection and ongoing monitoring, boosting market growth.
Oncology diagnostics play a crucial role in the early detection and management of various cancers. Additionally, the development of molecular diagnostics along with personalized medicine drives increasing demand for precise genetic mutation identification tests and cancer recurrence detection techniques, thus acting another growth-inducing factor.
Cardiology diagnostics requires diagnostic tools which include cholesterol testing alongside cardiac biomarker measurement and electrocardiogram (ECG) evaluation. Heart diseases maintain their status as major health problems, driving increased demand for advanced diagnostic methods to examine heart performance alongside risk determinants and disease evolution, which is catalyzing the market demand.
Nephrology diagnostics, which include tests for kidney function like serum creatinine, urine protein, and glomerular filtration rate (GFR), are vital for detecting kidney diseases. The rising prevalence of chronic kidney disease creates a need for improved diagnostic tools that detect kidney health problems at an early stage, thereby driving the market forward.
Analysis by End User:
- Diagnostic Laboratories
- Hospitals and Clinics
- Others
Diagnostic laboratories constitute South Korea's principal IVD market end user because they perform essential disease evaluation and disease management services. These laboratories depend on sophisticated IVD instruments combined with reagents to execute multiple diagnostic tests. Besides this, the laboratories serving patient diagnostic needs adopt up-to-date technologies to enhance both accuracy and efficiency while improving healthcare delivery.
The healthcare facilities of hospitals and clinics acquire IVD products to provide diagnosis and disease monitoring and treatment planning for patients. The healthcare industry requires increasingly accurate diagnostic tools that meet high standards because personalized medicine and early disease detection are taking precedence. This combination of laboratory-based and point-of-care diagnostics serves healthcare facilities to enhance clinical success while minimizing therapy expenses.
Regional Analysis:
- Seoul Capital Area
- Yeongnam (Southeastern Region)
- Honam (Southwestern Region)
- Hoseo (Central Region)
- Others
South Korea's healthcare and IVD market operates from the Seoul Capital Area which includes the capital city alongside bordering territories. The market growth in this area is propelled by its highly developed healthcare systems combined with high diagnostic service needs and its location of leading hospital and research organizations.
Yeongnam, the southeastern region of South Korea, has a strong industrial presence and expanding healthcare infrastructure. The growing healthcare investments combined with increasing healthcare needs transform Busan and Ulsan into significant markets for IVD products.
Honam, including cities like Gwangju and Jeonnam, is seeing steady growth in healthcare services and diagnostics. The local hospitals and clinics are demonstrating growing interest in IVD products because the region continues to draw additional healthcare investments.
Hoseo, covering areas like Daejeon and Chungcheong, has a developing healthcare sector with increasing demand for diagnostic services. As the region attracts more healthcare investments, there is rising interest in IVD products for improving diagnostic capabilities and healthcare delivery in local hospitals and clinics.
Competitive Landscape:
South Korea's in vitro diagnostics (IVD) market is highly competitive, featuring a diverse range of diagnostic solutions provided by both domestic and international companies. The market is characterized by rapid technological advancements, including innovations in molecular diagnostics, point-of-care testing, and automation. Companies are prioritizing product portfolio expansion, enhanced test accuracy, and faster turnaround times. The growing focus on personalized medicine is fueling the development of specialized diagnostic tools. Firms are adopting strategies such as strategic collaborations, partnerships, and increased investment in research and development to strengthen market positions and improve product offerings.
The report provides a comprehensive analysis of the competitive landscape in the South Korea in vitro diagnostics market with detailed profiles of all major companies.
Latest News and Developments:
- In January 2025, Fremman Capital acquired Diesse Diagnostica Senese from ArchiMed in a transaction valued at €125 million. ArchiMed's investors expect to achieve a 4.6 times multiple return on their initial investment through this business sale.
- In July 2024, Anbio Biotechnology unveiled a comprehensive range of IVD solutions at the 2024 Association for Diagnostics & Laboratory Medicine (ADLM) conference. The portfolio includes over 300 products applicable across various point-of-care testing scenarios, such as CLIA, FIA, RT-PCR, and lateral flow immunoassays.
- In November 2024, InSphero entered into a distribution partnership with Chayon to provide advanced 3D in vitro solutions for drug development to researchers in South Korea. This collaboration aims to support advancements in drug development and safety testing within the region.
- In January 2024, bioMérieux announced its acquisition of LUMED, a software company specializing in clinical decision support systems designed to optimize antimicrobial prescriptions and monitor healthcare-associated infections. This acquisition seeks to expand bioMérieux's capabilities in the IVD market.
- In August 2024, the China Association of In-Vitro Diagnostics (CAIVD) hosted a delegation from the Korea In Vitro Diagnostics Association (KIVDA) to strengthen ties between the IVD industries of both countries. The meeting focused on fostering cooperation and driving innovation within the sector.
South Korea In Vitro Diagnostics Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Billion USD |
| Scope of the Report |
Exploration of Historical and Forecast Trends, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Test Types Covered | Clinical Chemistry, Molecular Diagnostics, Immuno Diagnostics, Haematology, Others |
| Products Covered | Instrument, Reagent, Others |
| Applications Covered | Infectious Disease, Diabetes, Cancer/Oncology, Cardiology, Nephrology, Others |
| End Users Covered | Diagnostic Laboratories, Hospitals and Clinics, Others |
| Regions Covered | Seoul Capital Area, Yeongnam (Southeastern Region), Honam (Southwestern Region), Hoseo (Central Region), Others |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the South Korea in vitro diagnostics market from 2020-2034.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the South Korea in vitro diagnostics market.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the South Korea in vitro diagnostics industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
The South Korea in vitro diagnostics market was valued at USD 2.3 Billion in 2025.
Key factors driving the growth of the South Korea in vitro diagnostics (IVD) market include technological advancements, a growing demand for personalized medicine, an aging population, increased healthcare awareness, government initiatives supporting medical innovation, and the adoption of advanced diagnostic techniques in clinical settings.
IMARC estimates the South Korea in vitro diagnostics market to exhibit a CAGR of 5.45% during 2026-2034, reaching a value of USD 3.8 Billion by 2034.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












