
Relapsed Refractory Multiple Myeloma Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035
Market Overview:
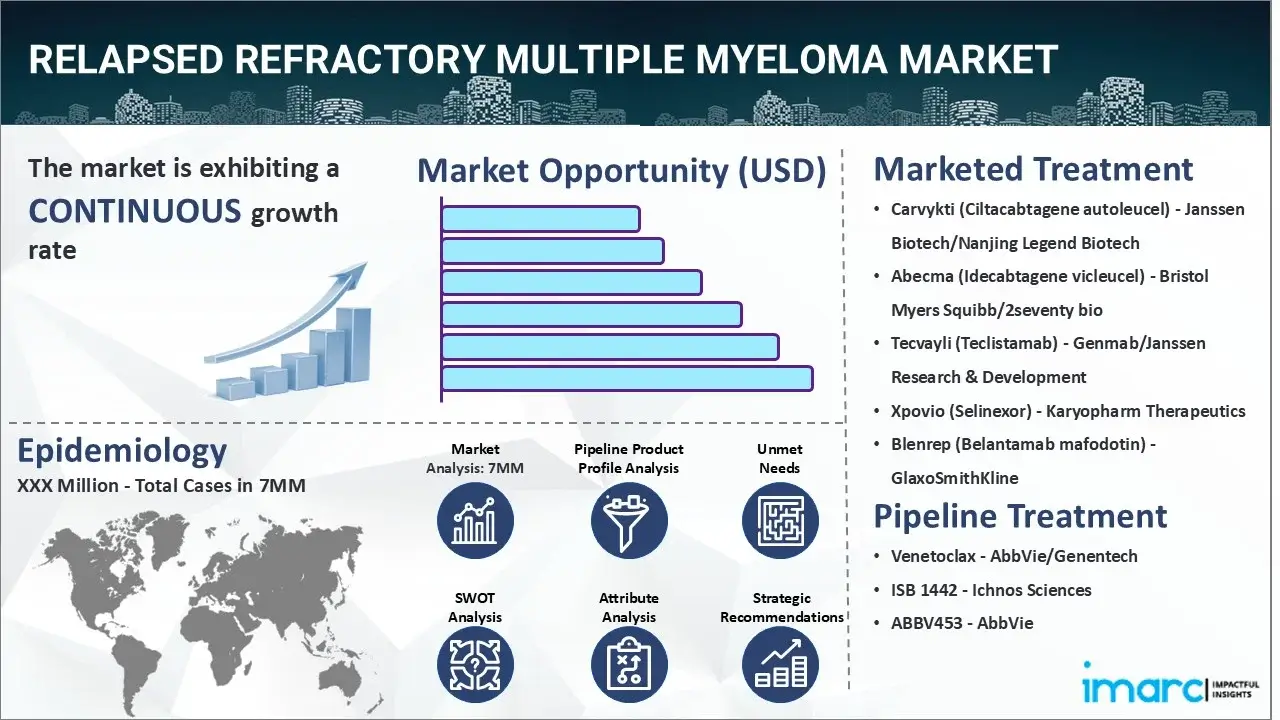
The relapsed refractory multiple myeloma market reached a value of USD 22.0 Billion across the top 7 markets (US, EU4, UK, and Japan) in 2024. Looking forward, IMARC Group expects the top 7 major markets to reach USD 37.3 Billion by 2035, exhibiting a growth rate (CAGR) of 4.95% during 2025-2035.
|
Report Attribute
|
Key Statistics
|
|---|---|
| Base Year | 2024 |
| Forecast Years | 2025-2035 |
| Historical Years |
2019-2024
|
|
Market Size in 2024
|
USD 22.0 Billion |
|
Market Forecast in 2035
|
USD 37.3 Billion |
|
Market Growth Rate 2025-2035
|
4.95% |
The relapsed refractory multiple myeloma market has been comprehensively analyzed in IMARC's new report titled "Relapsed Refractory Multiple Myeloma Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035". Relapsed refractory multiple myeloma, also known as RRMM, is a type of tumor that affects plasma cells, a form of white blood cell found in the bone marrow. This condition refers to a specific stage of the disease where the cancer has returned after a period of initial therapy and has become resistant to further treatment attempts. It is characterized by the presence of worsening symptoms, such as bone pain, fatigue, anemia, kidney problems, weakness, weight loss, lack of appetite, an increase in abnormal plasma cells in the bone marrow or other parts of the body, etc. An individual suffering from this illness may also experience recurrent infections, numbness in the limbs, difficulty with balance and coordination, changes in mental function, excessive thirst, nausea, confusion, constipation, etc. The diagnosis of RRMM is mainly based on a combination of medical history review, clinical feature assessment, and physical examination. Several imaging techniques, like X-rays, CT scans, MRIs, etc., are also utilized to identify the extent of the disease among patients. The healthcare provider may further perform a bone marrow biopsy to determine the presence and characteristics of cancerous plasma cells.
To get more information on this market, Request Sample
The increasing cases of genetic abnormalities, including chromosomal rearrangements and mutations in specific genes that can contribute to treatment resistance and disease recurrence, are primarily driving the relapsed-refractory multiple myeloma market. In addition to this, the rising prevalence of immune dysregulation, which blocks the function of T cells due to alterations in cytokine levels, is also creating a positive outlook for the market. Moreover, the widespread adoption of proteasome inhibitors, such as bortezomib, carfilzomib, ixazomib, etc., to selectively target and destroy the protein metabolism of abnormal plasma cells is further bolstering the market growth. Apart from this, the inflating application of antibody-drug conjugates that combine a monoclonal antibody with a potent anti-cancer drug to enhance the overall treatment response, thereby potentially leading to better outcomes for patients, is acting as another significant growth-inducing factor. Additionally, the escalating popularity of chimeric antigen receptor T-cell (CAR-T) therapy on account of its various advantages, including targeted treatment, long-term disease control, and improved efficacy, is expected to drive the relapsed-refractory multiple myeloma market during the forecast period.
IMARC Group's new report provides an exhaustive analysis of the relapsed-refractory multiple myeloma market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for relapsed refractory multiple myeloma and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the relapsed refractory multiple myeloma market in any manner.
Time Period of the Study
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the relapsed refractory multiple myeloma market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the relapsed refractory multiple myeloma market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report also provides a detailed analysis of the current relapsed refractory multiple myeloma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
| Drugs | Company Name |
|---|---|
| Carvykti (Ciltacabtagene autoleucel) | Janssen Biotech/Nanjing Legend Biotech |
| Abecma (Idecabtagene vicleucel) | Bristol Myers Squibb/2seventy bio |
| Tecvayli (Teclistamab) | Genmab/Janssen Research & Development |
| Xpovio (Selinexor) | Karyopharm Therapeutics |
| Blenrep (Belantamab mafodotin) | GlaxoSmithKline |
| Venetoclax | AbbVie/Genentech |
| ISB 1442 | Ichnos Sciences |
| ABBV453 | AbbVie |
*Kindly note that the drugs in the above table only represent a partial list of marketed/pipeline drugs, and the complete list has been provided in the report.
Key Questions Answered in this Report:
Market Insights
- How has the relapsed refractory multiple myeloma market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2024 and how are they expected to perform till 2035?
- What was the country-wise size of the relapsed refractory multiple myeloma across the seven major markets in 2024 and what will it look like in 2035?
- What is the growth rate of the relapsed refractory multiple myeloma across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of incident cases (2019-2035) of relapsed refractory multiple myeloma across the seven major markets?
- What is the number of incident cases (2019-2035) of relapsed refractory multiple myeloma by age across the seven major markets?
- What is the number of incident cases (2019-2035) of relapsed refractory multiple myeloma by gender across the seven major markets?
- What is the number of incident cases (2019-2035) of relapsed refractory multiple myeloma by type across the seven major markets?
- How many patients are diagnosed (2019-2035) with relapsed refractory multiple myeloma across the seven major markets?
- What is the size of the relapsed refractory multiple myeloma patient pool (2019-2024) across the seven major markets?
- What would be the forecasted patient pool (2025-2035) across the seven major markets?
- What are the key factors driving the epidemiological trend relapsed refractory multiple myeloma of?
- What will be the growth rate of patients across the seven major markets?
Relapsed Refractory Multiple Myeloma: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for relapsed refractory multiple myeloma drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the relapsed refractory multiple myeloma market?
- What are the key regulatory events related to the relapsed refractory multiple myeloma market?
- What is the structure of clinical trial landscape by status related to the relapsed refractory multiple myeloma market?
- What is the structure of clinical trial landscape by phase related to the relapsed refractory multiple myeloma market?
- What is the structure of clinical trial landscape by route of administration related to the relapsed refractory multiple myeloma market?
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization




.webp)




.webp)












