
Prefilled Syringes Market Size, Share, Trends and Forecast by Design, Material, Closing System, Application, End User, and Region, 2025-2033
Prefilled Syringes Market Overview:
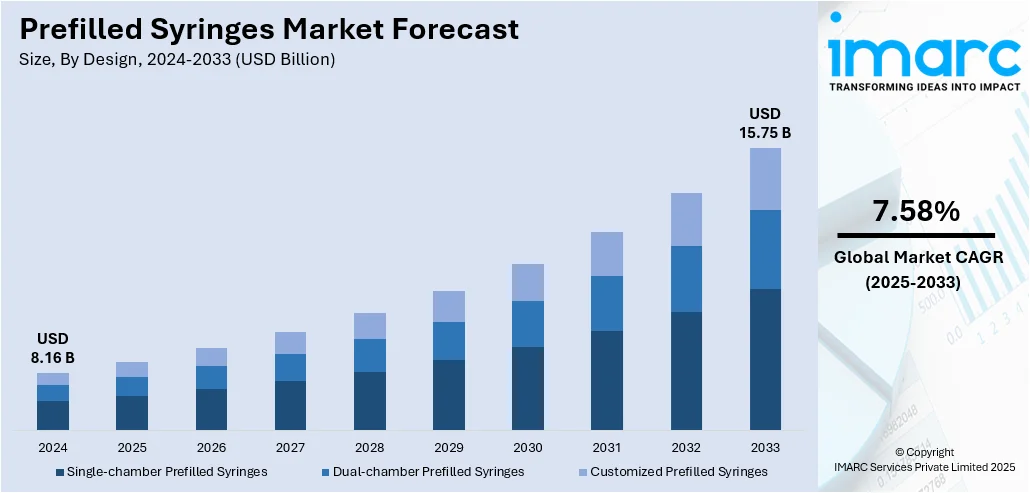
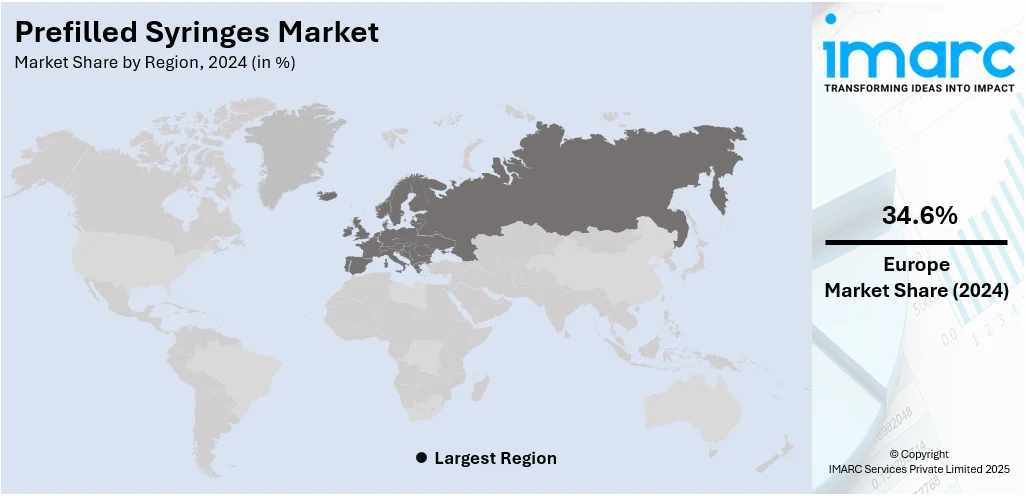
The global prefilled syringes market size was valued at USD 8.16 Billion in 2024. The market is projected to reach USD 15.75 Billion by 2033, exhibiting a CAGR of 7.58% from 2025-2033. Europe currently dominates the market, holding a market share of 34.6% in 2024. At present, increasing demand for biologics and the growing preferences for convenient and safe self-administration are promoting the adoption of advanced, contamination-free drug delivery systems like prefilled syringes. Besides this, the implementation of stringent regulatory standards is fueling the prefilled syringes market share.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 8.16 Billion |
|
Market Forecast in 2033
|
USD 15.75 Billion |
| Market Growth Rate 2025-2033 | 7.58% |
At present, the market is growing due to the rising demand for safe, easy, and accurate drug delivery. Prefilled syringes reduce the risk of contamination and medication errors, making them ideal for hospitals and home care. An increase in chronic diseases like diabetes and arthritis is creating the need for self-injection, encouraging more people to use prefilled syringes. Pharmaceutical companies also prefer them as they improve dosage accuracy and patient compliance. Moreover, advancements in materials and syringe design are making them more reliable and user-friendly. With rising awareness about hygiene and safety, along with an aging population needing regular treatment, the employment of prefilled syringes continues to increase across various healthcare settings.
To get more information on this market, Request Sample
The United States has emerged as a major region in the prefilled syringes market owing to many factors. Rising demand for efficient and safe drug delivery systems is fueling the prefilled syringes market growth. An increase in chronic diseases like diabetes, cancer, and autoimmune disorders is creating the need for regular injections, supporting the use of prefilled syringes. As per the NIH, in 2024, the count of new cancer cases was set to reach 2,001,140 in the United States. Prefilled syringes offer ease of use, reduce dosing errors, and lower the risk of contamination, making them popular in hospitals and home healthcare. The shift towards self-administration and patient-centered care is also catalyzing the demand. Pharmaceutical companies favor prefilled formats for biologic drugs, which require precise delivery.
Prefilled Syringes Market Trends:
Rising Demand for Injectable Drugs
Escalating demand for injectable drugs, especially biologics, vaccines, and therapeutic agents, is offering a favorable prefilled syringes market outlook. Injectable medications are often preferred for their fast-acting effects, crucial for chronic diseases, cancers, and autoimmune conditions, which require precise, controlled dosages. In line with this, prefilled syringes offer a convenient, ready-to-use solution that reduces the risks of dosage errors, contamination, and preparation time. Furthermore, the rising prevalence of chronic diseases and increased focus on patient-centric healthcare are driving the adoption of injectable drug formats, encouraging pharmaceutical companies to employ prefilled syringes for efficiency and safety. According to a 2024 report by the United States Centers for Disease Control and Prevention (CDC), around 129 Million individuals in the United States experienced at least one major chronic disease. Additionally, the US National Institutes of Health (NIH) estimated that by 2030, the global economic burden of chronic diseases is expected to reach USD 47 Trillion.
Growing Preferences for Safety and Convenience
Rising preferences for patient care and convenience are among the major prefilled syringes market trends. Healthcare providers and patients prefer prefilled syringes due to their enhanced safety features, including reduced contamination risks and accurate dosing. This format minimizes the risk of needlestick injuries, cross-contamination, and other hazards associated with traditional vials and syringes. In addition to this, with the growing emphasis on home healthcare and self-administration, prefilled syringes are becoming popular among patients, as they simplify the process, supporting ease of use and minimizing potential health risks. In 2024, the global home healthcare market reached USD 424.0 Billion, as per industry reports. The market is set to attain USD 816.4 Billion by 2033, growing at a CAGR of 7.48% during 2025-2033. This, in turn, is contributing to the market growth significantly.
Advancements in Prefilled Syringe Technology
Innovations in materials like the use of glass alternatives and ultra-thin needles are improving syringe durability and patient comfort. The development of safety-engineered devices and auto-disable syringes further ensures accurate dosing and prevents accidental misuse. Moreover, advancements in prefilled syringe manufacturing, such as automated filling and sealing processes, are enhancing product quality and scalability. For instance, in November 2024, Pharmaceutics International, Inc. (Pii), a contract development and manufacturing organization (CDMO) based in the US, revealed an investment of USD 3.6 Million to refine its prefilled syringe solutions, focusing on fill-finish processes, automated visual inspection, and advanced labeling. These technological improvements are essential as the pharmaceutical industry is shifting towards complex biologics that require specialized, sterile packaging, making advanced prefilled syringes the preferred choice for numerous therapeutic applications.
Prefilled Syringes Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global prefilled syringes market, along with forecast at the global, regional, and country levels from 2025-2033. The market has been categorized based on design, material, closing system, application, and end user.
Analysis by Design:
- Single-chamber Prefilled Syringes
- Dual-chamber Prefilled Syringes
- Customized Prefilled Syringes
Single-chamber prefilled syringes held 47.0% of the market share in 2024. They are preferred due to their simplicity, cost-effectiveness, and wide usage across various therapeutic areas. These syringes come preloaded with a fixed dose of medication, reducing preparation time and minimizing dosing errors. Their easy-to-use design makes them ideal for both healthcare professionals and patients, especially in emergency and self-administration situations. Single-chamber syringes also lower the risk of contamination, as there is no need for mixing or handling before utilization. They support better patient compliance, especially in chronic disease management, where regular injections are needed. Pharmaceutical companies are employing them because they are easier and cheaper to manufacture compared to dual-chamber systems. Their compatibility with a broad range of drugs, including vaccines and biologics, is further catalyzing the demand. As per the prefilled syringes market forecast, with healthcare systems moving towards safer and more efficient drug delivery methods, single-chamber prefilled syringes will continue to remain the top choice in the industry.
Analysis by Material:
- Glass Prefilled Syringes
- Plastic Prefilled Syringes
Glass prefilled syringes account for 72.5% of the market share. They hold dominance owing to their excellent barrier properties, chemical resistance, and long-standing reliability. Glass is highly compatible with a wide range of drugs, especially sensitive biologics, as it prevents interaction between the drug and the container, preserving purity and potency. It also offers better protection against oxygen and moisture, which is crucial for maintaining drug stability over time. Pharmaceutical companies prefer glass prefilled syringes because they support high sterility standards and are widely accepted by regulatory authorities. Glass syringes are strong, transparent, and allow easy visual inspection of the drug before administration. They are also heat-resistant, making them suitable for sterilization processes. Their proven performance, compatibility, and trust in the healthcare industry help maintain the dominant position in the market.
Analysis by Closing System:
- Staked Needle System
- Luer Cone System
- Luer Lock Form System
Staked needle system holds 43.6% of the market share. It is preferred due to its convenience, ease of use, and reduced risk of contamination. In this system, the needle is permanently attached to the syringe, eliminating the need for assembly before injection. This design simplifies the administration process, making it faster and more reliable for both healthcare professionals and patients, especially in emergency or home care settings. The staked needle system also ensures minimal drug wastage, as there is no dead space between the syringe and the needle. Its fixed structure improves dosing accuracy and enhances patient safety. Pharmaceutical companies prefer this system because it supports efficient filling and sealing during manufacturing. The staked needle design also lowers the risk of needle detachment or user error during handling. As the demand is rising for ready-to-use and safe injection devices, the staked needle system remains the most popular choice in the market.
Analysis by Application:
- Diabetes
- Anaphylaxis
- Rheumatoid Arthritis
- Oncology
- Others
Diabetes holds a significant share due to the growing number of patients who require regular insulin injections. Prefilled syringes offer a convenient, accurate, and sterile method for insulin delivery, especially for those who self-administer the drug. These syringes help reduce dosage errors and ensure patient compliance, which is crucial for effective diabetes management. Their ease of use and portability make them ideal for daily utilization.
Anaphylaxis is a serious, potentially fatal allergic response that necessitates prompt medical attention, typically with epinephrine. Prefilled syringes provide a quick, reliable solution in such emergencies, allowing fast administration without the need for preparation. Their ready-to-use design is critical for saving time during allergic reactions. Patients and caregivers often carry prefilled epinephrine syringes for emergency utilization, which increases their adoption.
Rheumatoid arthritis requires treatments that often involve regular subcutaneous injections. Prefilled syringes are ideal for these patients because they allow easy and accurate self-injection at home, reducing the need for frequent hospital visits. These syringes improve treatment adherence and offer comfort to individuals dealing with chronic joint pain and inflammation. Their precise dosing and sterile packaging enhance safety and convenience, making them a preferred option in long-term arthritis therapy.
In oncology, many cancer treatments involve injectable drugs that must be delivered with precision and under sterile conditions. Prefilled syringes support these needs by offering accurate dosages and reducing handling risks, which is especially important for patients with compromised immune systems. They also help streamline drug administration in both hospital and home care settings, improving workflow and minimizing treatment time.
Analysis by End User:
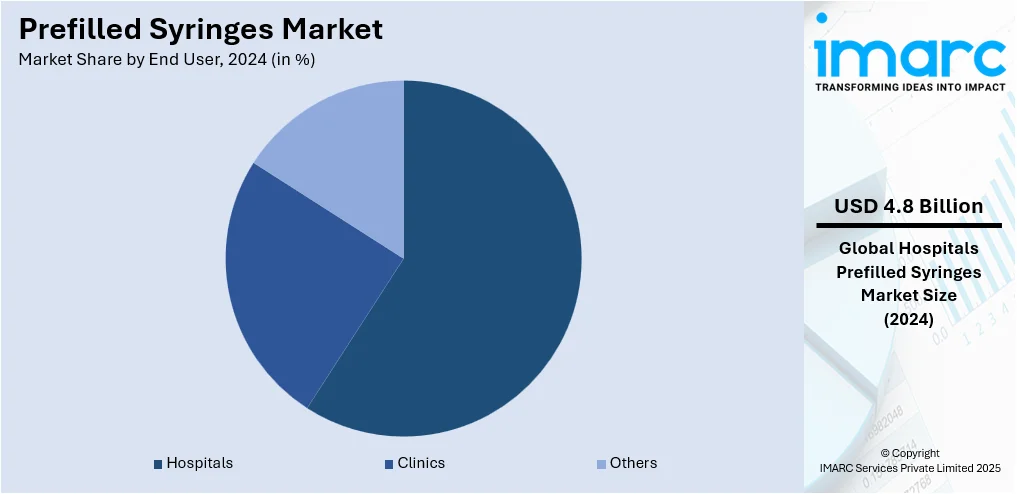
- Hospitals
- Clinics
- Others
Hospitals account for 59.3% of the market share. They handle a large volume of patients daily, and prefilled syringes support efficient workflows by offering ready-to-use solutions that save time for healthcare professionals. In hospital settings, prefilled syringes help reduce preparation time, minimize dosing errors, and lower the risk of contamination, which is critical in emergency care, surgeries, and routine treatments. These syringes also improve patient safety and enhance treatment accuracy, especially in cases requiring precise dosages. Hospitals frequently administer vaccines, biologics, and chronic disease medications, which are commonly delivered through prefilled syringes. The ability to maintain stringent hygiene standards and streamline inventory management further adds to their preference for prefilled systems. With a constant focus on patient care, safety, and operational efficiency, hospitals remain the largest and most consistent end users of prefilled syringes, leading this market segment by a significant margin.
Regional Analysis:
- North America
- United States
- Canada
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
Europe, accounting for a share of 34.6%, enjoys the leading position in the market. The region is noted for its strong healthcare infrastructure, advanced manufacturing capabilities, and high regulatory standards. The region has a large number of pharmaceutical and biotech companies that continuously innovate and produce high-quality injectable drugs, many of which are delivered through prefilled syringes. European countries are also promoting the use of safe and efficient drug delivery systems, which encourages hospitals and clinics to adopt prefilled syringes. Rising elderly population and the growing cases of chronic diseases are catalyzing the demand for regular injections. As per industry reports, the number of individuals aged 50 and older in England is expected to increase by 19.3% from 2024 to 2044, equating to an additional 4.3 Million people. The segment of the population that is 85 years and older is expanding the quickest. In addition, strong awareness about infection control and patient safety is promoting the utilization of prefilled syringes over traditional methods.
Key Regional Takeaways:
United States Prefilled Syringes Market Analysis
The United States holds 91.00% of the market share in North America. The North America prefilled syringes market is primarily driven by technological advancements, the growing use of biologics, and evolving healthcare practices. As biologic therapies, including monoclonal antibodies, vaccines, and hormone treatments, continue to rise in popularity, pharmaceutical companies are adopting prefilled syringes for their accuracy, sterility, and patient convenience. Innovations in syringe materials, such as advanced plastics and siliconization techniques, are enhancing performance, offering greater ease of use, and minimizing the risk of contamination compared to traditional vial-based systems. Additionally, the expansion of contract manufacturing and fill-finish outsourcing is boosting production capacity, enabling faster commercialization of biologic drugs and vaccines in prefilled formats across North America. For instance, in May 2025, PCI Pharma Services completed the purchase of Ajinomoto Althea, Inc, a subsidiary of the Japanese company Ajinomoto Co., Inc. This strategic acquisition would bolster Ajinomoto Althea's recent growth into sterile fill-finish, biologics, and advanced drug delivery systems, such as prefilled syringes. Other than this, self-administration trends are driving the demand, as patients with chronic ailments like rheumatoid arthritis and multiple sclerosis prefer the simplicity and portability of ready-to-use injection devices.
North America Prefilled Syringes Market Analysis
The North America prefilled syringes market is driven by a combination of healthcare, technological, and demographic factors that are driving the demand across multiple therapeutic areas. Rising prevalence of chronic diseases, such as diabetes, rheumatoid arthritis, and multiple sclerosis, is creating a strong need for convenient, ready-to-use drug delivery systems that improve patient adherence and reduce administration errors. The shift towards self-administration of injectable medications, especially in home healthcare settings, is further encouraging the use of prefilled syringes due to their ease of handling and reduced contamination risk. Technological advancements in syringe design, including refined safety features, enhanced material quality, and compatibility with biologics, are supporting the market growth. Additionally, increasing adoption of biologic and biosimilar drugs, which require injectable delivery, is catalyzing the demand. In April 2025, Regeneron and Roche declared the investment of over USD 53 Billion in the United States, focusing on extensive biologics production, cutting-edge gene therapy manufacturing, and the development of next-generation therapeutics. Stringent regulatory emphasis on minimizing needle-stick injuries and medication wastage is also influencing hospitals and healthcare providers to switch from traditional vials to prefilled options. The expansion of vaccination programs, particularly in response to infectious disease threats, and the region’s strong pharmaceutical manufacturing base are further accelerating the market penetration. Moreover, favorable reimbursement policies and the presence of key medical device firms continue to strengthen the market growth.
Europe Prefilled Syringes Market Analysis
The Europe prefilled syringes market is being propelled by the rapid growth of biologic and biosimilar drugs, which is catalyzing the demand for ready-to-use, high-quality injectable formats. As treatment is shifting towards at-home and community-based settings, the convenience and safety of prefilled syringes are being preferred over traditional vials, enabling self-administration for conditions, such as multiple sclerosis and rheumatoid arthritis. Pharmaceutical companies are also investing in advanced delivery technologies, including ultra-low dead-volume systems and silicone-free construction materials, to enhance drug stability and minimize dosing variability. Furthermore, manufacturers are scaling up production capabilities in Europe to support accelerated development timelines and decrease logistical complexity. For instance, in February 2025, Netherlands-based Tjoapack began the manufacturing of its inaugural prefilled syringe products for the delivery of Testosterone Cypionate in Europe. Tjoapack's PFS also inculcated the ability to assemble syringes with and without safety devices, in addition to managing automated procedures. Other than this, environmental concerns are driving innovations in eco-friendly packaging and recyclable components. As local production capacity is strengthening and development costs are distributed, market access is significantly expanding for smaller biotech firms.
Asia-Pacific Prefilled Syringes Market Analysis
The Asia-Pacific prefilled syringes market is expanding due to increasing healthcare investments and expanding health insurance coverage across developing nations. For instance, in India, healthcare spending in 2023-24 amounted to INR 5.85 Lakh Crores, showing a notable rise compared to 2017-18 at INR 2.43 Lakh Crores, according to the Press Information Bureau (PIB). As governments are prioritizing access to advanced therapies, the demand for efficient and reliable drug delivery systems is increasing. The growth of private hospitals and clinics is also encouraging the adoption of standardized, high-quality medical devices, such as prefilled syringes. Moreover, the broadening presence of multinational pharmaceutical companies in the region is promoting technology transfer and localized production, enhancing affordability and availability. With an aging population and rising incidence of chronic diseases, there is a heightened need for simplified drug administration methods that minimize dependency on healthcare professionals. Educational initiatives and training programs for both providers and patients are also improving confidence in prefilled syringes, supporting industry expansion.
Latin America Prefilled Syringes Market Analysis
The Latin America prefilled syringes market is experiencing robust growth due to the modernization of regional healthcare systems, which is promoting the adoption of ready-to-use delivery platforms to streamline hospital workflows and reduce preparation errors. Rising demand for biologic treatments and vaccine campaigns is also strengthening the infrastructure for prefilled formats. For instance, in August 2022, the Brazil Ministry of Health unveiled a National Vaccination Campaign to battle against various illnesses, such as poliomyelitis. With this release, approximately 40,000 immunization stations were made available nationwide to administer doses of the 18 various immunization types specified in the national vaccination plan. Besides this, governments are also implementing stringent standards on sterility and device safety, accelerating the shift away from traditional vials and supporting the market growth of prefilled syringes.
Middle East and Africa Prefilled Syringes Market Analysis
The Middle East and Africa prefilled syringes market is significantly influenced by the expansion of regional vaccination campaigns, catalyzing the demand for high-quality, single-use injection devices. Rising prevalence of chronic diseases, such as diabetes and autoimmune disorders, is also creating the need for self-administered biologic therapies, with prefilled syringes providing a safe and convenient delivery method. According to the International Diabetes Federation (IDF), in 2024, it was reported that 85 Million people in the Middle East and North Africa were affected by diabetes. This figure is expected to attain 163 Million by the year 2050. Other than this, the growth of private healthcare networks and home-care services in urban centers is also promoting the adoption of ready-to-use systems, facilitating industry expansion.
Competitive Landscape:
Key players are investing in research, innovations, and large-scale production. They are developing advanced syringe designs that improve safety, usability, and drug compatibility. These companies focus on enhancing materials and manufacturing processes to ensure high-quality and sterile products. They are also collaborating with pharmaceutical firms to offer customized solutions for biologics and other injectable drugs. Through strategic partnerships, acquisitions, and worldwide expansion, they are strengthening their market presence and reaching new customers. Key players also support regulatory compliance and quality standards, boosting trust among healthcare providers and patients. By offering a wide range of products, spending on automation, and promoting self-administration solutions, these companies help meet the growing healthcare demands, making them essential in the steady expansion of the prefilled syringes market. For instance, in September 2024, BD, a prominent global medical technology firm, revealed the commercial launch of the BD Neopak XtraFlow™ Glass Prefillable Syringe along with the capacity enhancement of the BD Neopak™ Glass Prefillable Syringe platform to address the increasing demand for biologic treatments.
The report provides a comprehensive analysis of the competitive landscape in the prefilled syringes market with detailed profiles of all major companies, including:
- B. Braun SE
- Becton, Dickinson and Company
- Catalent Inc.
- Fresenius Kabi AG
- Gerresheimer AG
- NIPRO Corporation
- SCHOTT Pharma AG & Co. KGaA
- Stevanato Group
- Taisei Kako Co., Ltd.
- West Pharmaceutical Services, Inc.
Latest News and Developments:
- June 2025: W. L. Gore & Associates, Inc. introduced the 0.5 mL silicone-free GORE IMPROJECT Syringe Plunger to the market. This innovative product was tailor-made for prefilled syringes utilized for multiple purposes, inculcating intravitreal injections for ocular ailments.
- May 2025: Zydus Lifesciences Limited obtained authorization from the US Food and Drug Administration (FDA) for its Glatiramer Acetate Injection, offered in 20 mg/mL and 40 mg/mL as single-dose prefilled syringes. This approval enhanced Zydus' position in launching advanced, distinctive generics to the market and reinforced the firm’s commitment to providing patients with diverse treatment choices.
- April 2025: The US Food and Drug Administration (FDA) granted approval to Argenx's VYVGART Hytrulo prefilled syringe (efgartigimod alfa and hyaluronidase-qvfc) for self-administration in adults diagnosed with generalized myasthenia gravis (gMG). The VYVGART Hytrulo prefilled syringe was approved for self-injection by a patient, caregiver, or healthcare professional as a subcutaneous injection, taking 20–30 seconds. Patients could self-administer injections once they were properly trained in the technique for subcutaneous injection.
- March 2025: ANI Pharmaceuticals, Inc. obtained FDA approval for its Purified Cortrophin® Gel (repository corticotropin injection USP) (Cortrophin Gel) presented in a prefilled syringe format. This groundbreaking prefilled syringe allowed patients to give Cortrophin Gel medication with considerably fewer steps. This authorization reflected the company's ongoing commitment to addressing the needs of those who utilized Cortrophin Gel therapy.
Prefilled Syringes Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Designs Covered | Single-chamber Prefilled Syringes, Dual-chamber Prefilled Syringes, Customized Prefilled Syringes |
| Materials Covered | Glass Prefilled Syringes, Plastic Prefilled Syringes |
| Closing Systems Covered | Staked Needle System, Luer Cone System, Luer Lock Form System |
| Applications Covered | Diabetes, Anaphylaxis, Rheumatoid Arthritis, Oncology, Others |
| End Users Covered | Hospitals, Clinics, Others |
| Regions Covered | North America, Asia-Pacific, Europe, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, China, Japan, India, South Korea, Australia, Indonesia, Germany, France, United Kingdom, Italy, Spain, Russia, Brazil, Mexico |
| Companies Covered | B. Braun SE, Becton, Dickinson and Company, Catalent Inc., Fresenius Kabi AG, Gerresheimer AG, NIPRO Corporation, SCHOTT Pharma AG & Co. KGaA, Stevanato Group, Taisei Kako Co., Ltd., West Pharmaceutical Services, Inc., etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 9-11 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the prefilled syringes market from 2019-2033.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the global prefilled syringes market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the prefilled syringes industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
The prefilled syringes market was valued at USD 8.6 Billion in 2024.
The prefilled syringes market is projected to exhibit a CAGR of 7.58% during 2025-2033, reaching a value of USD 15.75 Billion by 2033.
The increasing prevalence of chronic diseases, such as diabetes, rheumatoid arthritis, and cardiovascular conditions, is creating the need for self-administered injectable treatments, supporting the market growth. Pharmaceutical companies favor prefilled syringes for their ability to extend shelf life and enhance patient compliance. Additionally, advancements in biologics and biosimilars are catalyzing the demand for specialized delivery formats like prefilled syringes.
Europe currently dominates the prefilled syringes market, accounting for a share of 34.6% in 2024, due to strong healthcare systems, high regulatory standards, rising chronic disease cases, and advanced pharmaceutical manufacturing. Key players are investing in innovations, while the demand for safe, efficient, and self-administered drug delivery continues to rise.
Some of the major players in the prefilled syringes market include B. Braun SE, Becton, Dickinson and Company, Catalent Inc., Fresenius Kabi AG, Gerresheimer AG, NIPRO Corporation, SCHOTT Pharma AG & Co. KGaA, Stevanato Group, Taisei Kako Co., Ltd., West Pharmaceutical Services, Inc., etc.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












