
Pneumonia Testing Market Size, Share, Trends and Forecast by Type, Method, Technology, End User, and Region, 2025-2033
Pneumonia Testing Market Size and Share:
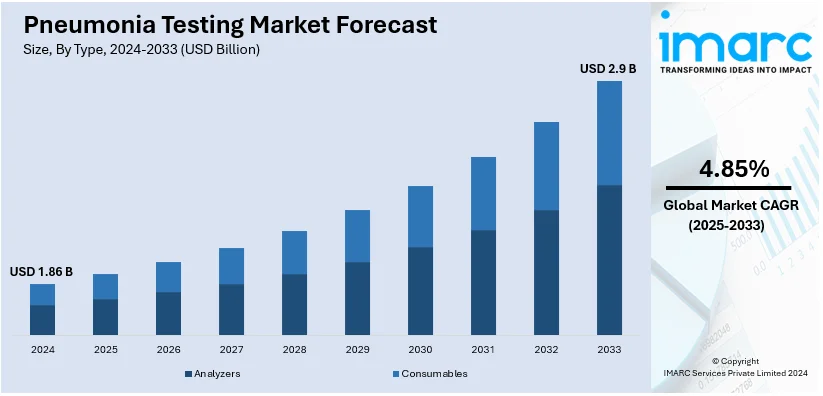
The global pneumonia testing market size was valued at USD 1.86 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 2.9 Billion by 2033, exhibiting a CAGR of 4.85% from 2025-2033. North America currently dominates the market, holding a market share of over 40.0% in 2024. The rising prevalence of pneumonia, technological advancements in diagnostics, increased healthcare spending and infrastructural improvements, and heightened awareness among the masses are some of the important factors supporting the market expansion.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024 |
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
| Market Size in 2024 | USD 1.86 Billion |
| Market Forecast in 2033 | USD 2.9 Billion |
| Market Growth Rate (2025-2033) | 4.85% |
Pneumonia continues to be a significant global health concern, particularly among vulnerable populations such as the elderly, children under five, and immunocompromised people. Based on recent findings by the WHO, pneumonia and diarrhoea together cause 21% of under-5 mortality and are responsible for around 1.1 million children’s deaths who are under 5. Recent data indicates a resurgence in certain types of pneumonia. For instance, mycoplasma pneumoniae infections have been increasing in 2024, with the percentage of pneumonia-associated emergency department visits with a mycoplasma-related diagnostic code peaking in August 2024 among children aged 2–4 years and 5–17 years. This rise in pneumonia cases underscores the growing demand for effective diagnostic solutions to facilitate timely and accurate detection, thereby driving the expansion of the pneumonia testing market.
The pneumonia testing market in the United States is growing rapidly with an 80.00% share. The country has witnessed 41,108 deaths in 2023 related to pneumonia. Furthermore, about 1.5 million adults in the US are diagnosed with pneumonia each year. Out of this, around 1 million are admitted to the hospital. This high incidence rate highlights the ongoing burden of pneumonia on the healthcare system, necessitating efficient and accurate testing methods to facilitate prompt treatment and reduce hospital admissions. Furthermore, the U.S. healthcare system's emphasis on early disease detection and preventive care has led to increased adoption of advanced diagnostic technologies. Government initiatives aimed at improving healthcare infrastructure and funding for infectious disease management further support the expansion of the pneumonia testing market.
Pneumonia Testing Market Trends:
Technological Advancements in Diagnostics
Advancements in pneumonia diagnostics have significantly enhanced the speed and accuracy of detection methods, contributing to improved patient outcomes. Recent developments in deep learning (DL) and artificial intelligence (AI) have been particularly impactful. For instance, convolutional neural networks (CNNs) have demonstrated high diagnostic accuracy in detecting childhood pneumonia from chest radiographs, with models like DenseNet201 and customized VGG16 achieving accuracies of 95% and 94%, respectively. The integration of federated learning with CNNs has further enhanced diagnostic capabilities. Studies have shown that federated learning models can achieve approximately 95% accuracy in pneumonia detection while effectively protecting patient data privacy. These technological advancements have not only improved diagnostic accuracy but also reduced the time required for diagnosis, enabling timely treatment interventions. As a result, the pneumonia testing market is experiencing growth, driven by the demand for advanced diagnostic solutions that leverage AI and machine learning technologies.
Increased Healthcare Spending and Infrastructure
Global healthcare expenditure has been on the rise, with significant investments directed toward improving healthcare infrastructure, particularly in emerging economies. This trend has facilitated better access to diagnostic services, including pneumonia testing. According to the World Economic Forum, spendings on health takes up to 10% of the global economy. Governments and private healthcare providers are investing heavily in healthcare infrastructure, particularly in emerging economies. Improved access to healthcare services, diagnostic centers, and laboratories is supporting the uptake of pneumonia testing solutions. Public health initiatives and funding for pneumonia prevention and diagnostics are also contributing to market expansion.
Rising Awareness and Screening Programs
Rising awareness and screening programs have tremendously influenced the global fight against pneumonia, especially through programmes like World Pneumonia Day, which is marked every November 12. In the year 2024, the "Pneumolight 2024" campaign lit up 228 monuments in 42 countries thereby increasing visibility and public engagement around pneumonia awareness. However, there is still an uphill task. Recently, a 2024 Irish survey reported that 77% of the population were unaware of or knew very little about pneumococcal disease, a common cause of pneumonia. Furthermore, 56% were also unaware that this disease is vaccine-preventable. This clearly indicates that the awareness of the public needs to be continued. Screening advancements have also been important. According to studies, machine learning (ML) models in emergency departments can contribute to the early detection of pneumonia, thus ensuring the timely treatment of patients.
Pneumonia Testing Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global pneumonia testing market, along with forecast at the global, regional, and country levels from 2025-2033. The market has been categorized based on type, method, technology, and end user.
Analysis by Type:
- Analyzers
- Consumables
Efficiency, accuracy, and the ability to process a high volume of samples in clinical settings have made analyzers dominate the pneumonia testing market. These instruments deliver results quickly and with high accuracy. This is what helps manage cases of pneumonia; immediate diagnosis directly influences the patient's outcome. Contemporary analyzers rely on automation and integration with laboratory information systems to streamline workflow and reduce human error. The demand for advanced analyzers is increasing because healthcare providers are focusing on faster turnaround times. Major players are investing in developing compact, high-throughput systems to cater to both centralized labs and decentralized facilities. Government initiatives to improve healthcare infrastructure in emerging economies also enhance market demand.
Analysis by Method:
- Immunodiagnostics
- Molecular Diagnostics
- Point of Care Testing
Point-of-care testing occupies the maximum share of pneumonia testing, currently at 42.7% owing to its quick results, convenience, and delivery of diagnostics at the patient's location. This evidence constitutes a major factor for pneumonia patients needing immediate treatment. It reduces delays in the diagnosis of pneumonia patients needing urgent treatment because POC testing is portable, easy to use, and test results are obtained on-site. The adopted forms of molecular diagnostics and antigen detection assays in POC formats have further cemented their role in pneumonia diagnosis.
Analysis by Technology:
- Enzyme-linked Immunosorbent Assay
- Immunofluorescence
- Western Bot
- Polymerase Chain Reaction
- Immunohistochemistry
- Others
Enzyme-linked immunosorbent assay or ELISA holds one of the highest market shares around29.6% of all pneumonia testing technologies. This characteristic is attributed to the high sensitivity, specificity, and cost-effectiveness of the method. Also, ELISA is applicable in the detection of diphtheritic bacterial, viral, and fungal antigens or antibodies; hence it is an important diagnostic method for pneumonia pathogens. There are several reasons why it is easily preferred in clinical labs and research laboratories: many samples can be done at once, and results are very precise as well as reliable. The latest technology has further enhanced the efficiency of the ELISA where automation has reduced the manual effort and much throughput has increased.
Analysis by End User:
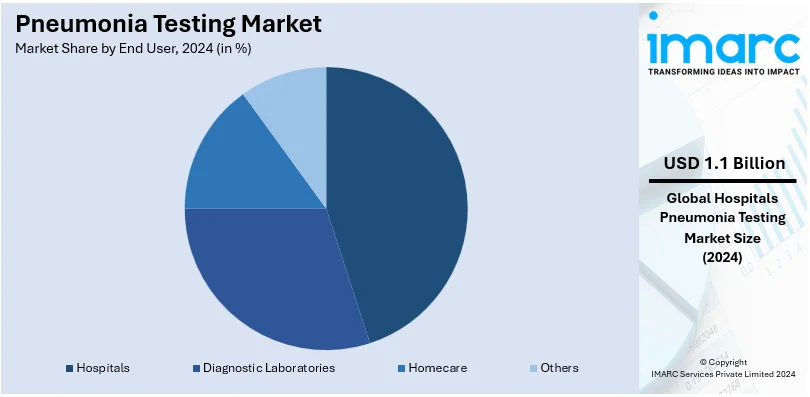
- Hospitals
- Diagnostic Laboratories
- Homecare
- Others
Hospitals dominate the pneumonia testing market, which is held at 59.8%. Hospitals remain the primary care centers that offer diagnosis and treatment services for severe cases. The facilities are equipped with highly sophisticated diagnostic equipment, including analyzers and molecular testing platforms, to offer thorough and accurate pneumonia testing. Rising pneumonia admissions among children, the elderly, and immunocompromised patients will result in hospitals' growing interest in advanced diagnostic solutions. These facilities also receive government funding, improved infrastructure, and skilled healthcare professionals. This further enhances their position in the market. Further, hospitals are using point-of-care testing and rapid diagnostic tools to help in quicker decision-making and reduced mortality rates.
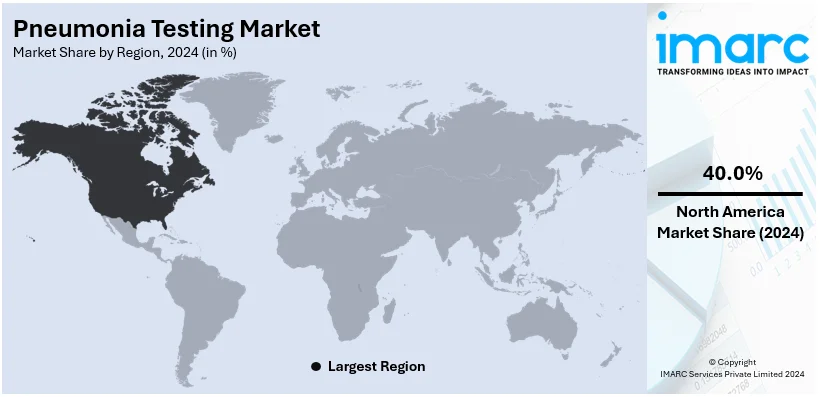
Regional Analysis:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
At 40.0%, North America claims the largest share of the pneumonia testing market in 2024. This is owing to the country's advanced healthcare infrastructure, serious investments in R&D, and increased awareness regarding early disease detection abilities. The region has a well-established diagnostic landscape with widespread adoption of advanced technologies like molecular diagnostics, enzyme-linked immunosorbent assays (ELISA), and point-of-care testing devices. The increasing prevalence of pneumonia in older and immunocompromised populations and government initiatives have contributed to this rapid growth in the market. Growing spending on healthcare and reimbursement policies will catalyze the deployment of advanced diagnostic solutions. Key market players in North America have been driving innovation by introducing automated analyzers and portable devices to enhance testing efficiency.
Key Regional Takeaways:
United States Pneumonia Testing Market Analysis
The pneumonia diagnostics market in the United States is primarily driven by the increasing incidence of pneumonia, particularly among chronic disease patients such as those suffering from heart conditions, diabetes, obesity, or hypertension. According to estimates provided by the US Department of Health and Human Services, a total of approximately 129 million Americans are afflicted with at least one major chronic condition, thereby placing them at risk for acquiring pneumonia. The highly developed health-care infrastructure of the country and overwhelming investments in medical technology would allow the development of early and precise diagnostic tests. Besides these, awareness regarding pneumonia-related complications and preventive healthcare exercises for both patients and providers would continue to support demand for timely diagnosis solutions. Furthermore, government initiatives that seek to eliminate pneumonia deaths, together with a robust reimbursement policy for diagnostic tests, will continue to energize this market. Viral pneumonia as well as pneumonia caused by viral respiratory infections, eg influenza and COVID-19, has resulted in demands for highly effective tests. Furthermore, the key players in the market continue innovating pneumonia diagnostics while the advancement in molecular diagnostics such as PCR and rapid antigen tests makes the testing more accurate and economical.
Europe Pneumonia Testing Market Analysis
The pneumonia testing market in Europe is growing rapidly owing to several factors. The EU population is estimated to be 448.8 Million on 1 January 2023, with more than one-fifth (21.3%) aged 65 years and over according to Eurostat. This aging demographic is a major driver of the demand for pneumonia diagnostics. The rising incidence of respiratory illnesses, especially in older adults, is increasing early and accurate detection needs around adults. There is also advanced healthcare systems and strong healthcare infrastructure in the region that increase adoption of advanced diagnostic technologies, such as molecular diagnostic techniques, PCR tests, and rapid antigen tests. The growing concern regarding antibiotic resistance also tends to increase the demand for precision tests which guide appropriate treatments. Government initiatives aimed at improving healthcare outcomes, along with an increasing focus on respiratory health management, also support market growth. The further increase is going to come from the emergence of point-of-care testing solutions and technological evolution of diagnostic devices. Lastly, the sound groundwork established by Europe in the manufacturing of medical devices is matched by its strict regulatory provisions to assure that all testing options for pneumonia will be of high quality. This creates an environment that is conducive to the continued development of this market.
Asia Pacific Pneumonia Testing Market Analysis
In the APAC region, the pneumonia testing market is driven by the high prevalence of respiratory infections, which affect approximately 475,000 individuals annually, accounting for 16.8% of the total disease burden, as reported by PubMed Central. Factors such as air pollution, changing climate conditions, and a growing elderly population exacerbate the incidence of pneumonia, driving demand for effective diagnostic solutions. Limited healthcare access in rural areas and emerging economies also creates a need for affordable and accessible testing options. Government initiatives aimed at improving healthcare infrastructure and increasing awareness about early diagnosis contribute to market growth. The rising prevalence of viral pneumonia, including infections like influenza and COVID-19, further accelerates the demand for pneumonia testing. Moreover, advancements in molecular diagnostics, such as PCR and rapid antigen tests, are gaining traction in the region, helping to improve the speed and accuracy of pneumonia detection. The expansion of private healthcare services and a focus on increasing access to care also play crucial roles in driving market growth across APAC.
Latin America Pneumonia Testing Market Analysis
In Latin America, the pneumonia testing market is primarily driven by the growing incidence of respiratory diseases and the need for accurate diagnostics to improve patient care. According to BMC Public Health, approximately 70% of Brazilians have at least one chronic disease by the age of 60, highlighting the significant burden of chronic conditions, especially among the aging population. This increases the vulnerability to pneumonia, further driving the demand for efficient diagnostic solutions. Healthcare reforms, government investments, and rising awareness of the importance of early detection contribute to the expansion of the pneumonia testing market in the region.
Middle East and Africa Pneumonia Testing Market Analysis
The pneumonia testing market in the Middle East and Africa is expanding due to increasing healthcare investments and rising awareness of respiratory diseases. The population at risk of chronic diseases, particularly those over 60, varies significantly across the region, ranging from 0.8% in the UAE to 10.6% in Turkey, as noted by DUPHAT. This growing demographic, coupled with the rising burden of infectious diseases, has heightened the need for accurate and rapid diagnostic solutions for pneumonia. Government efforts to improve healthcare access and control pneumonia outbreaks further support the adoption of advanced diagnostic technologies in the region.
Competitive Landscape:
Leading players in the pneumonia testing market are focusing on enhancing diagnostic accuracy, speed, and accessibility through technological advancements and strategic collaborations. They are investing heavily in research and development (R&D) to introduce advanced diagnostic solutions, such as molecular tests, rapid antigen detection kits, and AI-based imaging systems. These innovations aim to reduce diagnostic turnaround time, improve patient outcomes, and cater to the growing demand for decentralized testing, particularly in remote and resource-limited areas. Companies are also prioritizing point-of-care testing devices due to their portability and ability to deliver results in real-time, addressing the need for rapid diagnostics in critical care settings.
The report provides a comprehensive analysis of the competitive landscape in the pneumonia testing market with detailed profiles of all major companies, including:
- Abbott Laboratories
- Becton
- Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories Inc
- Curetis GmbH (OpGen)
- DiaSorin S.p.A.
- Hologic Inc
- Meridian Bioscience Inc
- Quest Diagnostics Incorporated
- Quidel Corporation
- Roche Holding AG
- Thermo Fisher Scientific Inc.
Latest News and Developments:
- November 2024: India introduced Nafithromycin, its first indigenously-developed antibiotic to combat antimicrobial resistance (AMR), offering advancements in diagnosing and managing drug-resistant pneumonia. The antibiotic addresses Community-Acquired Bacterial Pneumonia (CABP), a critical condition caused by resistant bacteria, with clinical trials confirming its effectiveness in a three-day regimen. This breakthrough supports improved pneumonia testing and targeted treatment, particularly for vulnerable populations such as children, the elderly, and immune-compromised individuals.
- November 2024: Abbott India introduced PneumoShield 14, a pneumococcal conjugate vaccine for children aged six weeks and above, designed to address the most extensive range of bacterial strains associated with pneumonia. It offers broader coverage compared to existing PCV-10 and PCV-13 vaccines from competitors like Pfizer, GSK, and Serum Institute of India.
- August 2024: Pfizer launched PfizerForAll, a digital platform to streamline healthcare access in the U.S. It offers same-day appointments, home delivery of medicines and diagnostic tests, and scheduling for pneumonia and other vaccinations. The platform also provides resources for insurance, savings, and patient support, addressing the need for simplified healthcare management.
- June 2024: Merck announced FDA approval of CAPVAXIVE™ (Pneumococcal 21-valent Conjugate Vaccine) for adults 18 and older, preventing invasive disease and pneumonia caused by 21 Streptococcus pneumoniae serotypes. The approval follows a Priority Review and addresses a major cause of invasive pneumococcal disease. CAPVAXIVE is not for individuals with severe allergic reactions to its components or diphtheria toxoid.
Pneumonia Testing Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Types Covered | Analyzers, Consumables |
| Methods Covered | Immunodiagnostics, Molecular Diagnostics, Point of Care Testing |
| Technologies Covered | Enzyme-linked Immunosorbent Assay, Immunofluorescence, Western Blot, Polymerase Chain Reaction, Immunohistochemistry, Others |
| End Users Covered | Hospitals, Diagnostic Laboratories, Homecare, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Abbott Laboratories, Becton, Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories Inc, Curetis GmbH (OpGen), DiaSorin S.p.A., Hologic Inc, Meridian Bioscience Inc, Quest Diagnostics Incorporated, Quidel Corporation, Roche Holding AG, and Thermo Fisher Scientific Inc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the pneumonia testing market from 2019-2033.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the global pneumonia testing market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the pneumonia testing industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
Pneumonia testing refers to the diagnostic procedures used to determine if someone has pneumonia, a lung infection that causes inflammation of the air sacs (alveoli). These tests help identify the cause of the infection, which can be bacterial, viral, or fungal, guiding proper treatment.
The pneumonia testing market was valued at USD 1.86 Billion in 2024.
IMARC estimates the global pneumonia testing market to exhibit a CAGR of 4.85% during 2025-2033.
The market expansion is supported by the rising prevalence of pneumonia, technological advancements in diagnostics, increased healthcare spending and infrastructural improvements, and heightened awareness among the masses.
In 2024, analyzer represented the largest segment by type, due to their efficiency, accuracy, and ability to process high sample volumes in clinical settings.
Point of care testing leads the market by method owing to its convenience, rapid results, and ability to deliver diagnostics at the patient’s location.
The enzyme-linked immunosorbent assay is the leading segment by technology due to its high sensitivity, specificity, and cost-effectiveness.
The hospitals are the leading segment by end user, as they are the primary healthcare providers for diagnosis and treatment of severe cases.
On a regional level, the market has been classified into North America, Asia Pacific, Europe, Latin America, and Middle East and Africa, wherein North America currently dominates the global market.
Some of the major players in the global pneumonia testing market include Abbott Laboratories, Becton, Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories Inc, Curetis GmbH (OpGen), DiaSorin S.p.A., Hologic Inc, Meridian Bioscience Inc, Quest Diagnostics Incorporated, Quidel Corporation, Roche Holding AG, Thermo Fisher Scientific Inc., etc.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












