
Obesity Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035
Market Overview:
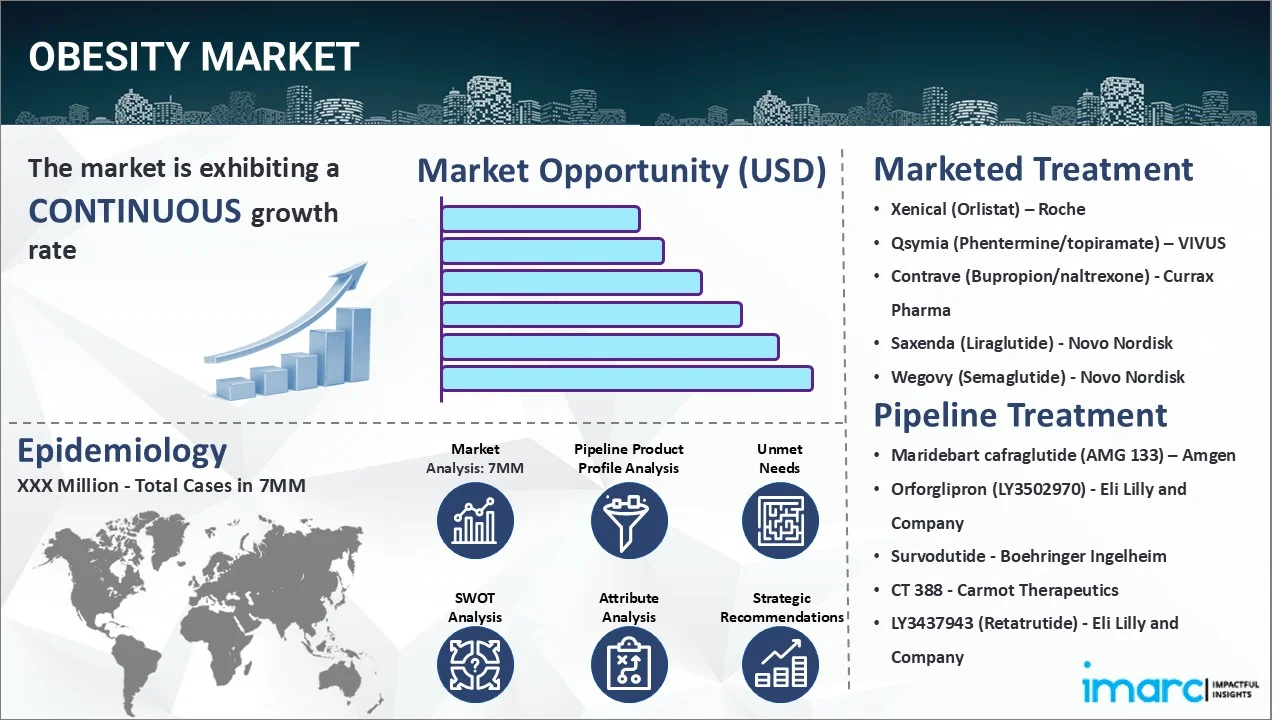
The 7 major obesity markets reached a value of USD 17.1 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 76.6 Million by 2035, exhibiting a growth rate (CAGR) of 14.62% during 2025-2035.
|
Report Attribute
|
Key Statistics
|
|---|---|
| Base Year | 2024 |
| Forecast Years | 2025-2035 |
| Historical Years |
2019-2024
|
|
Market Size in 2024
|
USD 17.1 Million |
|
Market Forecast in 2035
|
USD 76.6 Million |
|
Market Growth Rate 2025-2035
|
14.62% |
The obesity market has been comprehensively analyzed in IMARC's new report titled "Obesity Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035". Various researchers elucidate obesity as uncurbed growth of fat which results in various health issues. It is a long-term (chronic) health issue that worsens with time. Based on the definition given by Centers for Disease Control and Prevention (CDC), individuals having a body mass index (BMI) equal to 30 or higher are considered obese. Obese individuals are prone to several health conditions such as trouble while sleeping, gallstones, pain in joints, and other dermatological issues. The disorder can also cause several medical conditions related to heart, blood sugar, and range of liver disorders. Diagnosing obesity commonly involves a strict evaluation by a medical professional which not only includes a review of the patient’s medical history but also a physical assessment to measure the weight, height, and waist circumference of the patient. The healthcare provider may further perform blood tests, including lipid panels, thyroid function tests, blood sugar tests, and liver function tests.
To get more information on this market, Request Sample
The escalating cases of genetic and epigenetic mutations that can contribute to excess weight by altering the function of metabolic processes in the body, as well as regulating neural pathways and appetite centers, are primarily driving the obesity market. In addition to this, the inflating utilization of anti-obesity medications (AOMs), like liraglutide, orlistat, semaglutide, etc., to treat obesity by curbing appetite, delaying gastric emptying, or enhancing feelings of fullness, the market outlook is also positive. Furthermore, the extensive adoption of lifestyle modification, which includes a routine balanced diet and regular exercise to enhance self-esteem and enhancing overall well-being is further driving market growth. Apart from this, the rising usage of bariatric surgery, since it aids in weight loss by altering the digestive system to restrict food intake, decrease nutrient absorption, and alter hunger-regulating hormones, is acting as another significant growth-inducing factor. The growing adoption of gene therapy, which works by delivering genes that code for enzymes involved in fat breakdown to potentially improve metabolic health, is expected to drive the obesity market during the forecast period.
IMARC Group's new report provides an exhaustive analysis of the obesity market in the United States, EU5 (Germany, Spain, Italy, France, and United Kingdom) and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report the United States has the largest patient pool for obesity and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the obesity market in any manner.
Recent Developments:
In January 2025, Currax Pharmaceuticals LLC stated that it had launched its first broadcast TV ad and the "Cravings Don't Own Me" campaign. The commercial encourages people to seek help in taking control of their weight-loss attempts with CONTRAVE, an established drug that targets the emotion and appetite centers in the brain to lower hunger and regulate cravings, allowing them to achieve and maintain a healthy weight over time.
In November 2024, Amgen announced positive 52-week results from a double-blind, dose-ranging Phase 2 study of MariTide (maridebart cafraglutide, previously known as AMG 133), an investigational antibody-peptide conjugate administered via subcutaneous injection on a monthly or less frequent basis. The study demonstrated that MariTide led to an average weight loss of up to approximately 20% at week 52 in individuals with obesity or overweight who do not have Type 2 diabetes, suggesting the potential for sustained weight loss beyond 52 weeks.
In October 2024, VIVUS LLC announced that the FDA had approved a labeling update for QSYMIA (phentermine and topiramate extended-release capsules CIV). This update removed certain body mass index (BMI) requirements, along with warnings and precautions related to increased heart rate, the risk of hypoglycemia in individuals with type 2 diabetes on anti-diabetic therapy, and the potential for hypotension in those taking antihypertensive medications.
Drugs:
Xenical (Orlistat) is a lipase inhibitor that aids in obesity management by blocking the absorption of dietary fats. It acts by developing a covalent bond to the serine residue at the site of lipases (gastric and pancreatic) in the small intestine and stomach, thereby exerting its therapeutic effects. This inhibition prevents dietary triglycerides from being hydrolyzed into absorbable free fatty acids and monoglycerides. As a result, undigested triglycerides remain unabsorbed, creating a caloric deficit that may contribute to weight control.
Qsymia (Phentermine/Topiramate) is a combination medication containing phentermine and topiramate, designed for the treatment of obesity. Qsymia works largely by decreasing hunger through the action of phentermine, which is a stimulant and appetite suppressant. Phentermine increases norepinephrine levels in the brain, while topiramate potentially contributes to weight loss by modulating satiety signals and influencing taste perception.
Contrave (Bupropion/naltrexone) is an extended-release fixed-dose combination of naltrexone and bupropion. It is prescribed as an addition to a minimized-calorie diet and improved physical activity to manage weight in individuals whose BMI was 30 kg/m² or higher initially along with one other weight-related comorbidity. The naltrexone/bupropion combination affects hypothalamic brain regions that regulate hunger and energy expenditure, as well as regulating eating behavior through the reward system. The weight reduction caused by this drug in individuals is most likely due to these dual activities.
Maridebart cafraglutide (AMG 133), developed by Amgen, is a bispecific molecule that acts by simultaneously activating the glucagon-like peptide-1 (GLP-1) receptor and antagonizing the glucose-dependent insulinotropic polypeptide (GIP) receptor, thereby effectively promoting weight loss by mimicking the effects of GLP-1 while blocking the appetite-stimulating effects of GIP. Essentially, it works as a GLP-1 agonist and GIP antagonist, resulting in decreased food intake and improved metabolic metrics.
Orforglipron (LY3502970) is an investigational nonpeptide oral glucagon-like peptide-1 (GLP-1) receptor agonist currently being evaluated for long-term weight management in adults with obesity. It functions by activating the GLP-1 receptor, which regulates hunger, glucose metabolism, and weight control. Orforglipron stimulates this pathway to delay stomach emptying, enhance fullness, and decrease food intake. Furthermore, it lowers appetite by acting on the hypothalamus, reducing hunger signals.
Time Period of the Study
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the obesity market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the obesity market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report also provides a detailed analysis of the current obesity marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
| Drugs | Company Name |
|---|---|
| Xenical (Orlistat) | Roche |
| Qsymia (Phentermine/topiramate) | VIVUS |
| Contrave (Bupropion/naltrexone) | Currax Pharma |
| Saxenda (Liraglutide) | Novo Nordisk |
| Wegovy (Semaglutide) | Novo Nordisk |
| Maridebart cafraglutide (AMG 133) | Amgen |
| Orforglipron (LY3502970) | Eli Lilly and Company |
| Survodutide | Boehringer Ingelheim |
| CT 388 | Carmot Therapeutics |
| LY3437943 (Retatrutide) | Eli Lilly and Company |
*Kindly note that the drugs in the above table only represent a partial list of marketed/pipeline drugs, and the complete list has been provided in the report.
Key Questions Answered in this Report:
Market Insights
- How has the obesity market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2024 and how are they expected to perform till 2035?
- What was the country-wise size of the obesity market across the seven major markets in 2024 and what will it look like in 2035?
- What is the growth rate of the obesity market across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2019-2035) of obesity across the seven major markets?
- What is the number of prevalent cases (2019-2035) of obesity by age across the seven major markets?
- What is the number of prevalent cases (2019-2035) of obesity by gender across the seven major markets?
- How many patients are diagnosed (2019-2035) with obesity across the seven major markets?
- What is the size of the obesity patient pool (2019-2024) across the seven major markets?
- What would be the forecasted patient pool (2025-2035) across the seven major markets?
- What are the key factors driving the epidemiological trend of obesity?
- What will be the growth rate of patients across the seven major markets?
Obesity: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for obesity drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the obesity market?
- What are the key regulatory events related to the obesity market?
- What is the structure of clinical trial landscape by status related to the obesity market?
- What is the structure of clinical trial landscape by phase related to the obesity market?
- What is the structure of clinical trial landscape by route of administration related to the obesity market?
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization




.webp)




.webp)












