
North America HIV Drugs Market Size, Share, Trends and Forecast by Drug Class, Distribution Channel, and Country, 2025-2033
North America HIV drugs Market Size and Share:
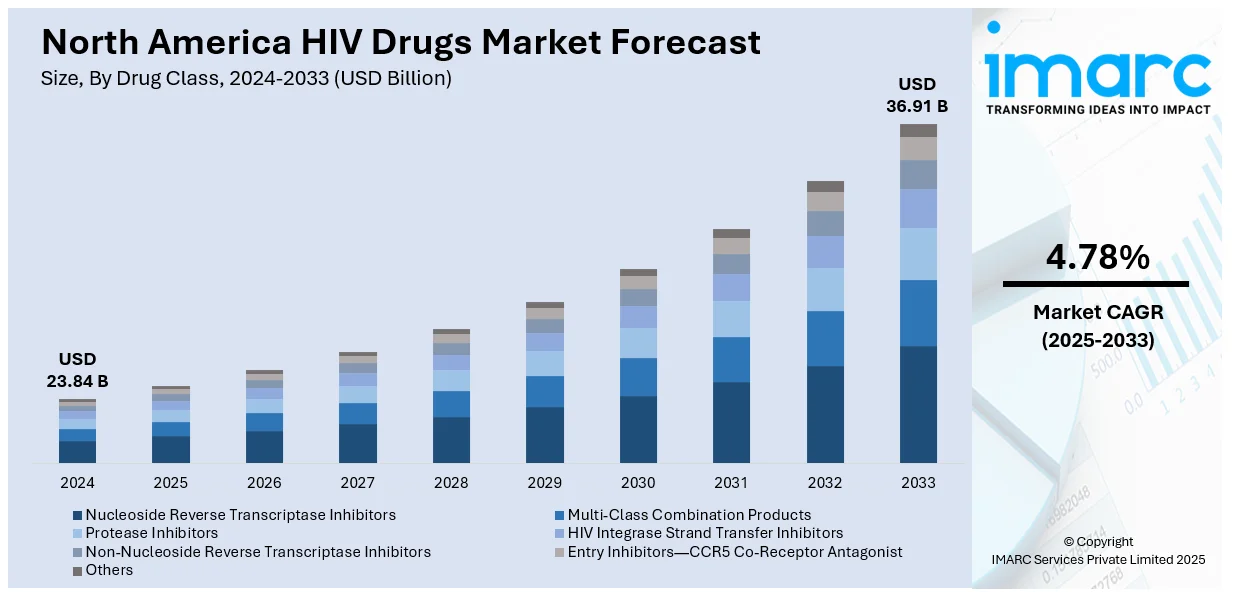
The North America HIV drugs market size was valued at USD 23.84 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 36.91 Billion by 2033, exhibiting a CAGR of 4.78% from 2025-2033. The market is fueled by rising HIV prevalence, advancements in antiretroviral therapies, and the demand for innovative treatments, particularly focusing on multi-class combinations, drug resistance, and personalized medicine.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 23.84 Billion |
|
Market Forecast in 2033
|
USD 36.91 Billion |
| Market Growth Rate (2025-2033) | 4.78% |
The North America HIV drugs market is influenced by the magnifying number of HIV diagnoses and the heightened demand for effective treatments. For instance, according to industry reports, in 2023, it is estimated that 2.3 million [2.0 million–2.7 million] people were living with HIV in the North America region. The increased availability of antiretroviral therapy (ART) has produced better care management for HIV patients, together with lower transmission rates and better health results. The market expansion is further supported by improved patient outcomes as a result of enhanced education initiatives regarding HIV and early disease detection and responsive treatment.
The continuous development of innovative therapies, such as long-acting injectables, is another key driver. These treatments aim to improve patient adherence by reducing the frequency of dosing, thus enhancing convenience and quality of life. Furthermore, ongoing research into drug resistance and personalized treatment options continues to shape the market, addressing the evolving needs of patients and fostering growth in the sector. For instance, in December 2024, the University of Saskatchewan's research on HIV immune responses reveals new interactions between APOBEC3 and Vif proteins, providing a better framework for drug design. This discovery offers hope for developing treatments that enhance the body’s natural defenses against HIV, potentially preventing infections.
North America HIV Drugs Market Trends:
Shift Towards Long-Acting Injectable HIV Medications
A notable trend in the North America HIV drugs market is the increasing adoption of long-acting injectable treatments. These therapies, designed to reduce the frequency of administration, improve patient adherence, and enhance convenience, are gaining significant traction. Long-acting formulations are particularly beneficial for patients who struggle with daily oral medications, offering a more practical alternative. This shift aligns with the growing demand for patient-centered treatments, as it allows individuals to manage their condition with fewer hospital visits. As research and development continue, new injectable options are expected to expand, creating a significant impact on the market. For instance, in December 2024, Gilead Sciences submitted a New Drug Application to the FDA for lenacapavir as a twice-yearly injectable HIV-1 prevention treatment. Its phase 3 trials showed 99.9% efficacy in preventing HIV and superior results compared to daily oral Truvada.
Emphasis on Personalized Medicine
Personalized medicine is emerging as a prominent trend in the North America HIV drugs market. With advancements in genetic profiling and molecular diagnostics, healthcare providers are increasingly tailoring HIV treatments to individual patients. This approach ensures that therapies are optimized based on a patient’s specific genetic makeup and resistance profiles. For instance, in February 2024, researchers from the University of Waterloo in Canada developed a pH-sensitive RNAi-based nanomicrobicide for preventing vaginal HIV transmission. The siRNA-loaded nanoparticles targeted CCR5 and Nef genes, demonstrating effective gene knockdown, autophagy reactivation, and HIV replication inhibition, with promising results in both in vitro and in vivo models. Personalized medicine enhances treatment efficacy and reduces the likelihood of adverse side effects, offering a more targeted and effective treatment plan. As the understanding of HIV’s molecular mechanisms improves, the market is expected to see a rise in customized therapies, which can lead to better long-term outcomes for patients.
Rising Focus on Drug-Resistant HIV Strains
Drug resistance continues to be a growing concern in the North America HIV drugs market, driving the development of new therapies targeting resistant strains of the virus. As HIV patients undergo long-term treatment, the emergence of drug-resistant variants presents a significant challenge. To address this, pharmaceutical companies are focusing on developing next-generation antiretrovirals that are effective against resistant strains. For instance, as per industry reports, it is estimated that 77% of individuals living with HIV in the region in 2023 are on antiretroviral therapy (ART), primarily consisting of adults aged between 57 and 91. This trend is crucial for maintaining the effectiveness of HIV treatments and ensuring long-term disease control. As drug resistance patterns evolve, the market will likely witness an increased demand for innovative treatments that target resistant HIV strains, thereby supporting continued North America HIV drugs market growth.
North America HIV Drugs Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the North America HIV drugs market, along with forecasts at the country and regional levels from 2025-2033. The market has been categorized based on drug class and distribution channel.
Analysis by Drug Class:
- Nucleoside Reverse Transcriptase Inhibitors
- Multi-Class Combination Products
- Protease Inhibitors
- HIV Integrase Strand Transfer Inhibitors
- Non-Nucleoside Reverse Transcriptase Inhibitors
- Entry Inhibitors—CCR5 Co-Receptor Antagonist
- Others
Multi-class combination products are emerging as the leading drug class segment in the North America HIV drugs market. These products combine multiple antiretroviral agents from different classes, offering enhanced efficacy, reduced pill burden, and improved patient adherence to treatment regimens. By targeting various stages of the HIV life cycle, multi-class combinations provide comprehensive viral suppression and reduce the risk of resistance. The convenience of once-daily dosing and reduced side effects further contribute to their growing adoption. As a result, multi-class combination products are expected to play a pivotal role in the continued advancement of HIV treatment strategies in North America.
Analysis by Distribution Channel:
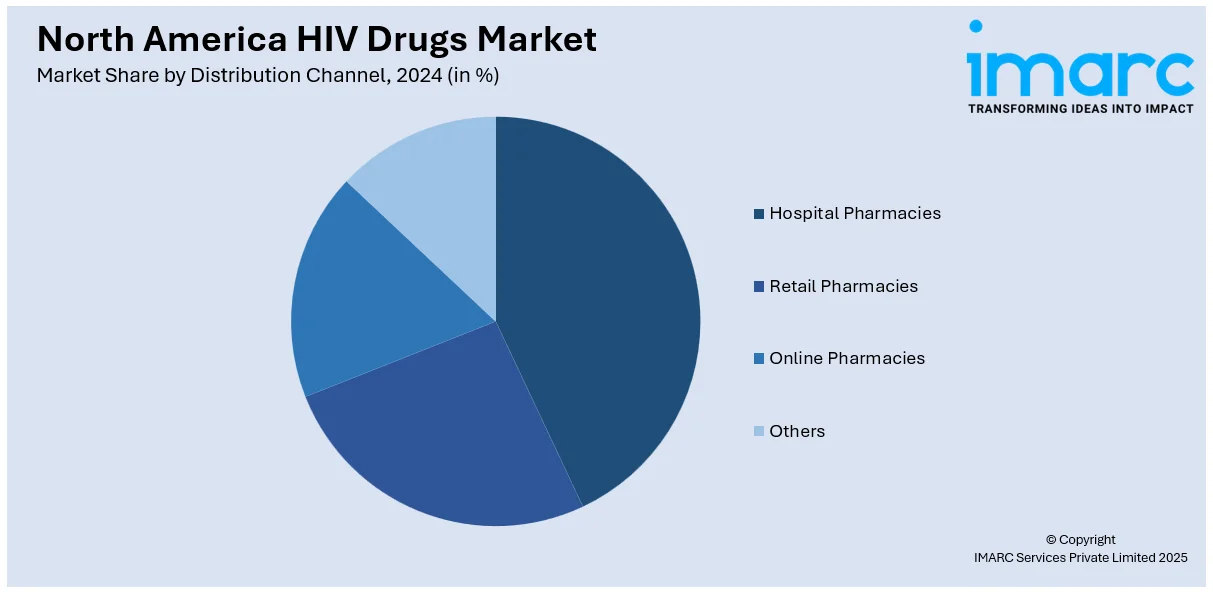
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Others
Hospital pharmacies are the leading distribution channel segment for the North America HIV drugs market. These pharmacies deliver specialized HIV care including antiretroviral therapies (ART) to treat patients who reside in hospital facilities. In addition, hospital pharmacies maintain trained staff members who provide precise medication dispensing while offering drug counseling as well as tracking treatment medications. Moreover, the strategic placement of these pharmacies enables healthcare systems to manage HIV treatment efficiently for patients who need advanced treatment procedures and have drug-resistant strains. Besides this, the advancing field of HIV care depends on the fundamental role played by hospital pharmacies to maintain optimal drug access and treatment success.
Country Analysis:
- United States
- Canada
The United States is the leading country segment in the North America HIV drugs market, holding a significant share of the North American HIV drugs market share. With a high HIV prevalence rate and advanced healthcare infrastructure, the U.S. provides broad access to antiretroviral therapies (ART) and innovative HIV treatments. Moreover, government initiatives, such as the Ending the HIV Epidemic (EHE) plan, help patients gain better access to care while decreasing transmission rates and enhancing treatment results. As per official reports, the initiative aims to decrease new HIV infections in the United States by 75% by 2025, with a further reduction of at least 90% by 2030. The U.S. also serves as a center for clinical trials and HIV drug development, contributing to continuous advancements in treatment options and strengthening its dominance in the regional market.
Competitive Landscape:
The North America HIV drugs market presents competitive dynamics with diverse pharmaceutical companies developing sophisticated antiretroviral therapies (ART). The market remains highly dynamic because pharmaceutical companies intensely focus their research on multi-class combination products and next-generation treatments. Pharmaceutical businesses emphasize drug efficiency together with enhanced patient results and solutions for drug resistance development. In addition, organizations along with public sector entities utilize strategic collaboration, partnership and merger to develop their product lines and improve market reach. For instance, in July 2023, Gilead Sciences collaborated with CHAI, Penta ID, and Monell to develop pediatric HIV treatments, including dispersible F/TAF formulations and bitterness-reducing agents, aiming to enhance medication adherence and access in low-income countries. Besides this, the increasing focus on personalized medicine and targeted HIV strain therapies accelerates competition among pharmaceutical corporations working to create better accessible therapeutic solutions.
The report provides a comprehensive analysis of the competitive landscape in the North America HIV drugs market with detailed profiles of all major companies.
Latest News and Developments:
- In May 2024, ViiV Healthcare announced Health Canada’s approval of APRETUDE (cabotegravir tablets and injectable suspension) for pre-exposure prophylaxis (PrEP). This long-acting injectable option, requiring just six injections per year, expands access to effective HIV prevention for at-risk individuals in Canada.
- In July 2024, amfAR, the American Foundation for AIDS Research, announced new grants for researchers focused on eliminating the HIV reservoir, a key barrier to a cure. These projects aim to provide valuable insights into overcoming latent HIV and advancing cure research.
North America HIV Drugs Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report |
Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Drug Classes Covered | Nucleoside Reverse Transcriptase Inhibitors, Multi-Class Combination Products, Protease Inhibitors, HIV Integrase Strand Transfer Inhibitors, Non-Nucleoside Reverse Transcriptase Inhibitors, Entry Inhibitors—CCR5 Co, Receptor Antagonist, Others |
| Distribution Channels Covered | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies, Others |
| Countries Covered | United States, Canada |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the North America HIV drugs market from 2019-2033.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the North America HIV drugs market.
- Porter's Five Forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the North America HIV drugs industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Key Questions Answered in This Report
The North America HIV drugs market was valued at USD 23.84 Billion in 2024.
The market is influenced by rising HIV diagnoses, increased demand for effective treatments, and advancements in antiretroviral therapy (ART). Additionally, growing awareness, government initiatives, and a focus on personalized medicine and drug resistance management are fueling market expansion, improving patient outcomes, and enhancing disease control.
IMARC estimates the North America HIV drugs market to reach USD 36.91 Billion in 2033, exhibiting a CAGR of 4.78% during 2025-2033.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












