
Multiple System Atrophy Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035
Market Overview:
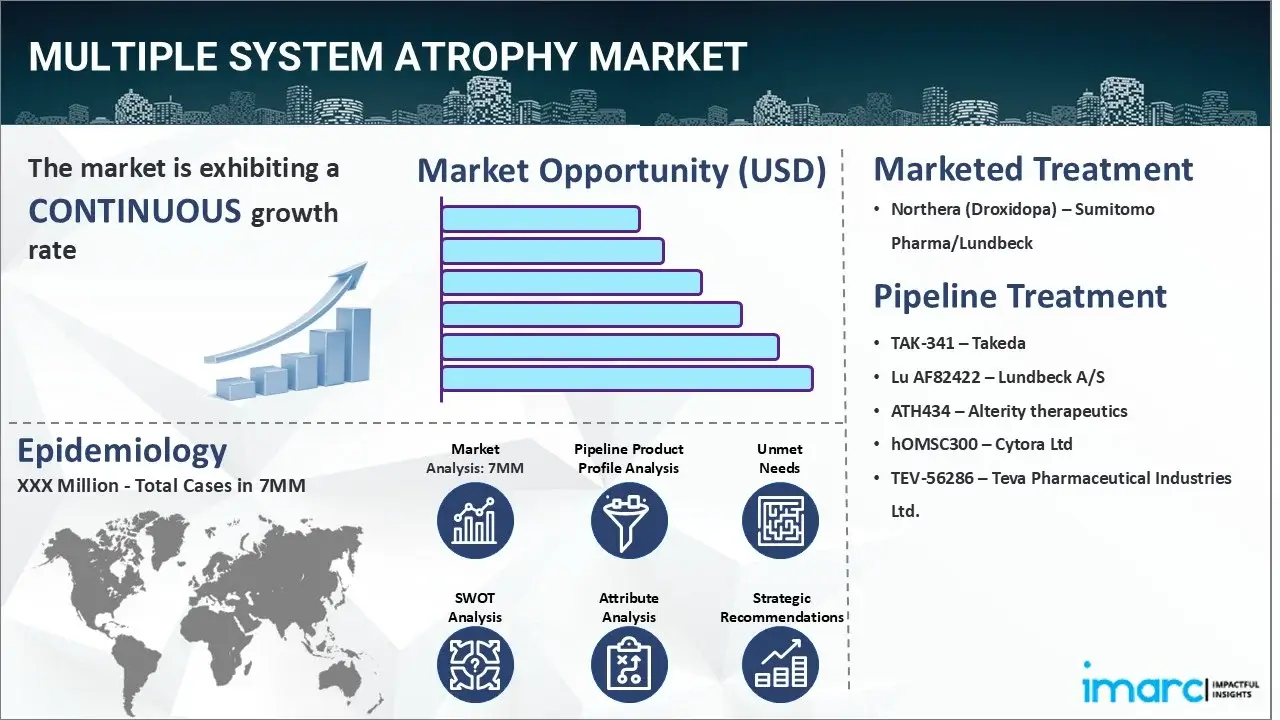
The multiple system atrophy market reached a value of USD 133.8 Million across the top 7 markets (US, EU4, UK, and Japan) in 2024. Looking forward, IMARC Group expects the top 7 major markets to reach USD 200.0 Million by 2035, exhibiting a growth rate (CAGR) of 3.74% during 2025-2035.
|
Report Attribute
|
Key Statistics
|
|---|---|
| Base Year |
2024
|
| Forecast Years | 2025-2035 |
| Historical Years |
2019-2024
|
|
Market Size in 2024
|
USD 133.8 Million |
|
Market Forecast in 2035
|
USD 200.0 Million |
|
Market Growth Rate (2025-2035)
|
3.74% |
The multiple system atrophy market has been comprehensively analyzed in IMARC's new report titled "Multiple System Atrophy Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook". Multiple system atrophy (MSA) is a rare and progressive neurodegenerative disorder that generally impacts the autonomic nervous system and the motor system. The symptoms of MSA primarily include a combination of indications such as parkinsonism (tremors, stiffness, and slow movements), loss of balance and coordination, difficulty speaking and swallowing, problems with bladder and bowel control, and changes in blood pressure regulation that cause fainting spells. Due to its resemblance to other neurological disorders and the lack of precise diagnostic testing, diagnosing MSA can be difficult. A conclusive diagnosis of MSA usually requires a combination of clinical, neurological, and imaging evaluations. A clinical evaluation includes a detailed medical history, physical examination, and an assessment of the patient's motor function, balance, speech, and cognition. A neurological assessment consists of tests, such as a spinal tap, electroencephalography, and electromyography. Numerous imaging studies, including MRI and PET scans, are utilized to help identify changes in the brain that are characteristic of MSA.
To get more information on this market, Request Sample
The rising prevalence of neurogenerative disorders along with the increasing unmet clinical need for treatment is primarily driving the global multiple system atrophy market. In addition to this, the introduction of specific diagnostic criteria, such as the MSA consensus criteria, which help to enhance the accuracy of MSA diagnosis and reduce the number of misdiagnoses, is also creating a positive outlook for the market. Besides this, the emerging popularity of non-pharmacological approaches, such as physiotherapy, occupational therapy, and speech therapy, to assist in managing the symptoms and improving the quality of life for people with MSA is also bolstering the global market. Furthermore, several technological advancements in the field of neuroscience, including the introduction of advanced imaging technologies and gene editing, are augmenting the development of novel diagnostic tools and treatments for MSA. This, in turn, is acting as a significant growth-inducing factor. Apart from this, extensive investments in research activities aimed at analyzing the potential of stem cell therapy for regenerating damaged neurons and improving disease symptoms are expected to drive the global multiple system atrophy market in the coming years.
IMARC Group's new report provides an exhaustive analysis of the multiple system atrophy market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for multiple system atrophy and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario and unmet medical needs, etc. have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the multiple system atrophy market in any manner.
Time Period of the Study
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the multiple system atrophy market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the multiple system atrophy market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report also provides a detailed analysis of the current multiple system atrophy marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
| Drugs | Company Name |
|---|---|
| Northera (Droxidopa) | Sumitomo Pharma/Lundbeck |
| TAK-341 | Takeda |
| Lu AF82422 | Lundbeck A/S |
| ATH434 | Alterity therapeutics |
| hOMSC300 | Cytora Ltd |
| TEV-56286 | Teva Pharmaceutical Industries Ltd. |
*Kindly note that the drugs in the above table only represent a partial list of marketed/pipeline drugs, and the complete list has been provided in the report.
Key Questions Answered in this Report:
Market Insights
- How has the multiple system atrophy market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2024 and how are they expected to perform till 2035?
- What was the country-wise size of the multiple system atrophy across the seven major markets in 2024 and what will it look like in 2035?
- What is the growth rate of the multiple system atrophy across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2019-2035) of multiple system atrophy across the seven major markets?
- What is the number of prevalent cases (2019-2035) of multiple system atrophy by age across the seven major markets?
- What is the number of prevalent cases (2019-2035) of multiple system atrophy by gender across the seven major markets?
- What is the number of prevalent cases (2019-2035) of multiple system atrophy by type across the seven major markets?
- How many patients are diagnosed (2019-2035) with multiple system atrophy across the seven major markets?
- What is the size of the multiple system atrophy patient pool (2019-2024) across the seven major markets?
- What would be the forecasted patient pool (2025-2035) across the seven major markets?
- What are the key factors driving the epidemiological trend multiple system atrophy of?
- What will be the growth rate of patients across the seven major markets?
Multiple System Atrophy: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for multiple system atrophy drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the multiple system atrophy market?
- What are the key regulatory events related to the multiple system atrophy market?
- What is the structure of clinical trial landscape by status related to the multiple system atrophy market?
- What is the structure of clinical trial landscape by phase related to the multiple system atrophy market?
- What is the structure of clinical trial landscape by route of administration related to the multiple system atrophy market?
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization




.webp)




.webp)












