
Kaposi's Sarcoma Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035
Market Overview:
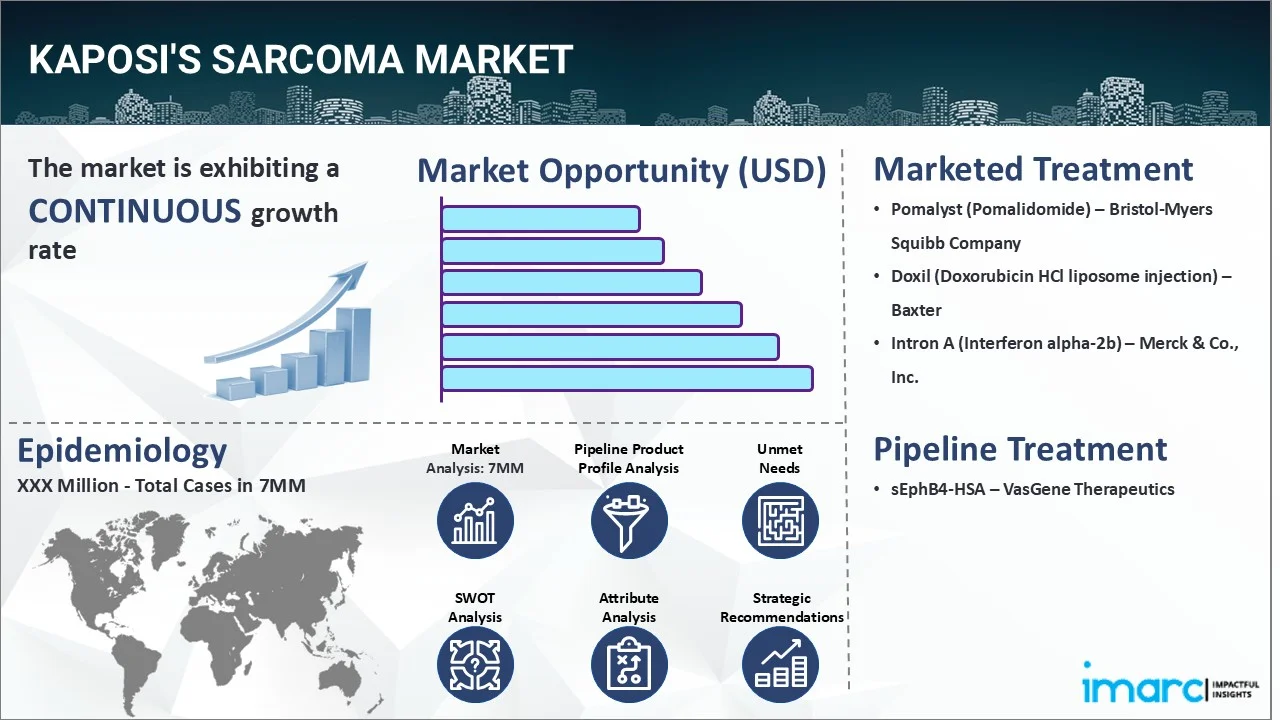
The kaposi's sarcoma market reached a value of USD 113.3 Million across the top 7 markets (US, EU4, UK, and Japan) in 2024. Looking forward, IMARC Group expects the top 7 major markets to reach USD 169.9 Million by 2035, exhibiting a growth rate (CAGR) of 3.78% during 2025-2035.
|
Report Attribute
|
Key Statistics
|
|---|---|
| Base Year |
2024
|
| Forecast Years | 2025-2035 |
| Historical Years |
2019-2024
|
|
Market Size in 2024
|
USD 113.3 Million |
|
Market Forecast in 2035
|
USD 169.9 Million |
|
Market Growth Rate (2025-2035)
|
3.78% |
The Kaposi's sarcoma market has been comprehensively analyzed in IMARC's new report titled "Kaposi's Sarcoma Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035". Kaposi's sarcoma (KS) refers to a type of cancer that affects the cell linings of the blood and lymph vessels. The symptoms of the ailment depend on the location and extent of the lesions. The most typical indication is the development of red or purple patches and nodules on the skin or mucous membranes, which may be painless or tender. These lesions can vary in size as well as number and may be flat or raised. The condition can also affect internal organs, leading to symptoms such as shortness of breath, chest pain, abdominal pain, nausea, vomiting, etc. In cases of HIV-associated Kaposi's sarcoma, the disease may be accompanied by other indications of advanced HIV infection, including fever, night sweats, weight loss, fatigue, etc. The diagnosis involves a physical examination and a biopsy of the affected tissue. The biopsy sample is examined under a microscope to look for the characteristic spindle-shaped cells that are seen in KS. Several additional procedures are performed to determine the extent of the ailment, such as imaging studies like X-rays, CT scans, or MRI scans. Various blood tests are also advised to check for infection with Kaposi's sarcoma-associated herpesvirus.
To get more information on this market, Request Sample
The increasing cases of human herpesvirus 8 (HHV-8) infections that affect the cell linings of blood vessels and lymphatic vessels, leading to the formation of lesions, are primarily driving the Kaposi's sarcoma market. Furthermore, the widespread adoption of targeted therapy drugs, such as bevacizumab or pazopanib, to target specific proteins or pathways that are involved in the development of the ailment is acting as another significant growth-inducing factor. Additionally, the inflating utilization of photodynamic therapy as a treatment option, which uses a special light and a photosensitizing drug to destroy cancer cells, is further augmenting the market growth. Moreover, the escalating demand for topical treatments, including imiquimod cream and retinoids, to treat lesions on the skin or mucous membranes caused by KS is also creating a positive outlook for the market. Apart from this, the introduction of numerous advanced imaging techniques, such as magnetic resonance imaging (MRI) and positron emission tomography (PET), which have greatly improved the ability to detect as well as monitor lesions in various parts of the body, including soft tissues, bones, lymph nodes, etc., is expected to drive the Kaposi's sarcoma market in the coming years.
IMARC Group's new report provides an exhaustive analysis of the Kaposi’s sarcoma market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for Kaposi’s sarcoma and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the Kaposi’s sarcoma market in any manner.
Key Highlights:
- In the United States, around 2,000 cases of Kaposi sarcoma are reported each year or 6 cases per million people.
- The major group in the United States that develops Kaposi sarcoma is the posttransplant population, with a frequency of roughly 1 in 200.
- In Africa, the annual incidence of Kaposi sarcoma is quite high, with 37.7 per 100,000 men and 20.5 per 100,000 women.
- Classic Kaposi sarcoma mainly affects older males of Mediterranean and Eastern European descent.
- Classic Kaposi sarcoma primarily affects men, with a male-to-female ratio of 10-15:1.
Time Period of the Study
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Kaposi's sarcoma market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Kaposi's sarcoma market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report also provides a detailed analysis of the current Kaposi's sarcoma marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
| Drugs | Company Name |
|---|---|
| Pomalyst (Pomalidomide) | Bristol-Myers Squibb Company |
| Doxil (Doxorubicin HCl liposome injection) | Baxter |
| Intron A (Interferon alpha-2b) | Merck & Co., Inc. |
| sEphB4-HSA | VasGene Therapeutics |
*Kindly note that the drugs in the above table only represent a partial list of marketed/pipeline drugs, and the complete list has been provided in the report.
Key Questions Answered in this Report:
Market Insights
- How has the Kaposi’s Sarcoma market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2024 and how are they expected to perform till 2035?
- What was the country-wise size of the Kaposi’s Sarcoma across the seven major markets in 2024 and what will it look like in 2035?
- What is the growth rate of the Kaposi’s Sarcoma across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of incident cases (2019-2035) of Kaposi’s Sarcoma across the seven major markets?
- What is the number of incident cases (2019-2035) of Kaposi’s Sarcoma by age across the seven major markets?
- What is the number of incident cases (2019-2035) of Kaposi’s Sarcoma by gender across the seven major markets?
- What is the number of incident cases (2019-2035) of Kaposi’s Sarcoma by type across the seven major markets?
- How many patients are diagnosed (2019-2035) with Kaposi’s Sarcoma across the seven major markets?
- What is the size of the Kaposi’s Sarcoma’ patient pool (2019-2024) across the seven major markets?
- What would be the forecasted patient pool (2025-2035) across the seven major markets?
- What are the key factors driving the epidemiological trend of Kaposi’s Sarcoma?
- What will be the growth rate of patients across the seven major markets?
Kaposi's Sarcoma: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Kaposi’s Sarcoma drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Kaposi’s Sarcoma market?
- What are the key regulatory events related to the Kaposi’s Sarcoma market?
- What is the structure of clinical trial landscape by status related to the Kaposi’s Sarcoma market?
- What is the structure of clinical trial landscape by phase related to the Kaposi’s Sarcoma market?
- What is the structure of clinical trial landscape by route of administration related to the Kaposi’s Sarcoma market?
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization




.webp)




.webp)












