
Indian Vaccine Market Size, Share, Trends and Forecast by Monovalent and Combined Vaccines, 2026-2034
Indian Vaccine Market Summary:
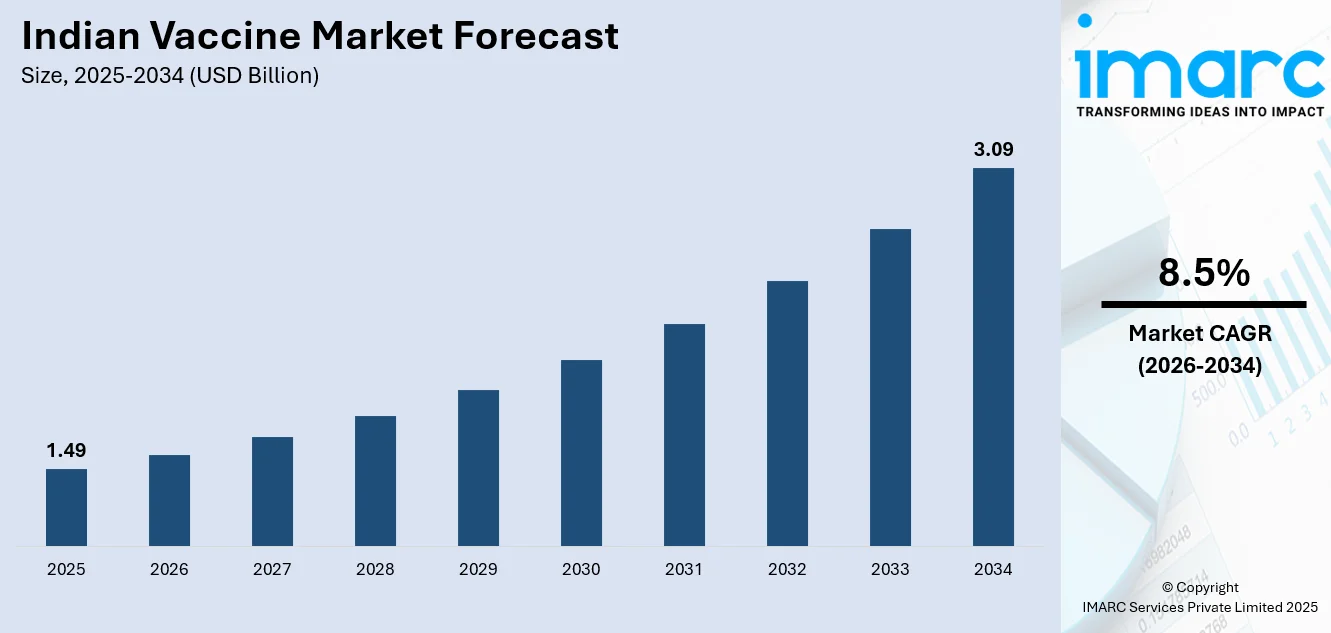
The Indian vaccine market size was valued at USD 1.49 Billion in 2025 and is projected to reach USD 3.09 Billion by 2034, growing at a compound annual growth rate of 8.5% from 2026-2034.
The Indian vaccine market is experiencing substantial momentum driven by the government's emphasis on preventive healthcare and robust domestic manufacturing capabilities. Rising public awareness about immunization benefits, expanded healthcare access in rural regions, and continuous technological advancements in vaccine development are strengthening market expansion. The Universal Immunization Programme's comprehensive coverage, strategic international collaborations, and India's position as a global vaccine manufacturing hub are collectively driving Indian vaccine market share.
Key Takeaways and Insights:
- By Monovalent and Combined Vaccines: Oral polio vaccine (OPV) leads the market with a share of 8% in 2025, driven by India's sustained polio elimination efforts and the Universal Immunization Programme's comprehensive coverage targeting millions of newborns annually.
- Key Players: The Indian vaccine market exhibits strong competitive dynamics with established domestic manufacturers leading production volumes alongside multinational pharmaceutical corporations. Companies are expanding manufacturing capacities, diversifying vaccine portfolios across pediatric, adult, and travel segments, and forming strategic international partnerships to strengthen market presence and drive innovation. Some of the key players are GlaxoSmithKline, Sanofi Aventis, Serum Institute of India, Panacea Biotec, Pfizer, Novartis, VHB Lifesciences, Zydus Cadila, and MSD.
To get more information on this market Request Sample
The Indian vaccine market is advancing rapidly as the nation reinforces its position as the global leader in vaccine manufacturing. India produces approximately 60% of the world's vaccine supply, with the Serum Institute of India alone maintaining an annual production capacity of 4 billion doses at its Manjri facility in Pune. India’s Universal Immunization Programme provides free vaccination against multiple preventable diseases for newborns and pregnant women, strengthening public health nationwide. The country’s regulatory framework for vaccines meets global standards, ensuring safety and international confidence. Government initiatives like Mission Indradhanush have expanded immunization coverage across districts, while digital health platforms such as the U-WIN portal support real-time tracking of vaccine administration, registration of pregnant women and children, and efficient management of nationwide immunization efforts.
Indian Vaccine Market Trends:
Rising Government Initiatives and Immunization Programs
Government-led vaccination initiatives continue advancing India's preventive healthcare landscape through comprehensive coverage expansion and infrastructure development. The Universal Immunization Programme receives substantial funding, with INR 12,300 crore allocated in 2024 to strengthen vaccination coverage across underserved regions. Mission Indradhanush's twelve completed phases have vaccinated over 5.46 crore children and 1.32 crore pregnant women, systematically addressing immunization gaps in hard-to-reach areas. The U-WIN portal, launched nationally in 2024, enables digital registration and tracking across more than 185,000 healthcare facilities, transforming vaccination record management and supporting Indian vaccine market growth.
Expansion of Domestic Manufacturing and International Collaborations
India's vaccine manufacturing ecosystem is witnessing significant capacity expansion through strategic domestic investments and global partnerships. In January 2024, CEPI announced a USD 30 million investment in Serum Institute of India to expand outbreak vaccine production capabilities as part of the 100 Days Mission for pandemic preparedness. Technology transfer agreements for vaccine manufacturing are strengthening India’s pharmaceutical infrastructure, enhancing the country’s capacity to produce vaccines for both domestic use and global distribution. Such collaborations support international health security efforts while expanding access to critical immunization solutions worldwide.
Technological Innovation and Digital Health Integration
Technology is transforming India's vaccine ecosystem through innovations in development platforms, delivery mechanisms, and supply chain management. The Electronic Vaccine Intelligence Network (eVIN) digitizes vaccine logistics over 29,000 cold chain points, enabling real-time temperature monitoring and stock management nationwide. Indian manufacturers are advancing next-generation vaccine platforms, including mRNA and intranasal technologies, with Bharat Biotech's iNCOVACC becoming the world's first intranasal COVID-19 vaccine. In May 2024, Serum Institute acquired a 20% stake in IntegriMedical to advance needle-free injection technology, potentially revolutionizing vaccine administration and improving patient compliance across diverse population segments.
Market Outlook 2026-2034:
The Indian vaccine market is poised for strong growth, fueled by broader immunization coverage, technological innovations, and the country’s expanding role in global vaccine production. Government efforts, including the expansion of immunization programs and the adoption of digital health platforms, are improving vaccine accessibility in both urban and rural areas. Increasing public awareness of healthcare, the rising burden of infectious diseases, and growing demand for adult and travel vaccines are creating additional opportunities, positioning the market for sustained expansion and greater participation in both domestic and international immunization efforts. The market generated a revenue of USD 1.49 Billion in 2025 and is projected to reach a revenue of USD 3.09 Billion by 2034, growing at a compound annual growth rate of 8.5% from 2026-2034.
Indian Vaccine Market Report Segmentation:
| Segment Category | Leading Segment | Market Share |
|---|---|---|
| Monovalent and Combined Vaccines | Oral Polio Vaccine (OPV) | 8% |
Monovalent and Combined Vaccines Insights:
- BCG
- HIB
- Influenza
- Varicella
- Typhoid
- Japanese Encephalitis
- Measles
- Tetanus Toxoid
- Hepatitis A
- Rubella
- Diphtheria, Tetanus, and Pertussis (DPT)
- Oral Polio Vaccine (OPV)
- MMR
- Rotavirus
- Hepatitis B
- Pneumococcal
- Meningococcal
- Rabies
- HPV
- Hexavalent
- Dengue vaccines
Oral polio vaccine (OPV) leads the market with a share of 8% of the total Indian vaccine market in 2025.
The oral polio vaccine continues to represent a leading position in the market due to India’s ongoing focus on polio prevention and routine immunization efforts. Its oral administration offers key advantages, including ease of use in large-scale vaccination campaigns, minimal reliance on trained healthcare personnel, and higher compliance in rural and hard-to-reach communities. Continued vigilance in vaccination ensures sustained protection against polio and supports broader public health goals, reinforcing the vaccine’s importance in preventive healthcare strategies. For instance, in April 2025, Bharat Biotech announced a collaboration with Bilthoven Biologicals for oral polio vaccine production and supply, strengthening domestic manufacturing capabilities.
The Universal Immunization Programme's comprehensive coverage ensures OPV accessibility across all districts, with Pulse Polio campaigns maintaining high vaccination rates among children under five years. The government's National Immunization Schedule mandates multiple OPV doses during infancy, creating sustained demand throughout the year. India's manufacturing expertise in oral vaccine formulations, combined with cost-effective production capabilities, positions domestic manufacturers as key suppliers for both national programs and international procurement agencies including WHO and UNICEF.
Market Dynamics:
Growth Drivers:
Why is the Indian Vaccine Market Growing?
Government Immunization Programs and Policy Support
India's extensive government-led immunization initiatives are fundamentally driving vaccine market expansion through comprehensive coverage mandates and substantial funding allocations. The Universal Immunization Programme, one of the world's largest public health initiatives, targets around 26 million newborns and 34 million pregnant women annually with free vaccinations against various diseases. Mission Indradhanush has played a key role in expanding immunization coverage across the country, driving broader access to essential vaccines. Complementing this, digital platforms like U-WIN have established comprehensive registries for children and vaccine tracking, enhancing efficiency and transparency in immunization management. Continued government support and investment in vaccination infrastructure are strengthening healthcare delivery, particularly in underserved and remote regions, ensuring more consistent access to preventive healthcare and reinforcing the nation’s commitment to public health.
Rising Burden of Infectious Diseases and Preventive Healthcare Awareness
The high prevalence of infectious diseases in India, combined with rising public awareness of preventive healthcare, is driving strong demand for vaccines across all age groups. Increased understanding of vaccination’s role in preventing serious illnesses is shifting consumer behavior toward proactive health measures. India’s demographic profile, with a substantial pediatric population and a growing number of older adults susceptible to conditions such as influenza and pneumococcal infections, further sustains vaccine demand. Awareness initiatives and emphasis on cost-effective preventive care are reinforcing the importance of immunization in public health strategies nationwide.
Expansion of Domestic Manufacturing and Export Capabilities
India’s strong vaccine manufacturing capabilities and growing production capacity are enhancing domestic supply while boosting its competitiveness in global markets. Leading manufacturers maintain large-scale production facilities, enabling consistent vaccine availability and supporting international distribution to numerous countries, reinforcing India’s role as a key player in global immunization efforts. For instance, in October 2025, CEPI announced USD 16.4 million funding for Serum Institute to develop H5N1 bird flu vaccines using baculovirus platform technology as part of pandemic preparedness efforts. Bharat Biotech has established a USD 75 million integrated Cell and Gene Therapy facility in Hyderabad's Genome Valley, announced in March 2025, expanding advanced manufacturing capabilities. The government's Production Linked Incentive scheme has encouraged pharmaceutical companies to enhance production capacities, addressing both domestic needs and international vaccine demands through technology transfers and strategic partnerships.
Market Restraints:
What Challenges the Indian Vaccine Market is Facing?
Cold Chain Infrastructure Limitations
Despite significant investments, gaps in cold chain infrastructure continue to impact vaccine distribution, especially in remote and rural areas. Power interruptions, limited refrigeration, and challenging geography make it difficult to maintain temperature-controlled storage, leading to spoilage and inefficiencies. Addressing these issues requires ongoing modernization of infrastructure and adoption of sustainable solutions such as solar-powered refrigeration to ensure vaccines remain effective throughout the supply chain.
Vaccine Hesitancy and Misinformation
Vaccine hesitancy, driven by misinformation, safety concerns, and cultural beliefs, persists as a barrier to the fulfillment of comprehensive immunization coverage. Fears about side effects and vaccine effectiveness, and rumors perpetuated through social media, make the public wary. Lower levels of digital literacy and limited access to accurate information in rural areas make it even harder to build trust and confidence in the benefits of vaccination.
Regulatory and Supply Chain Constraints
Regulatory compliance needs and vulnerabilities in supply may at times dent vaccine availability and market access timelines. Supply chain vulnerabilities arise due to the limited capacity for local battery manufacturing and dependence on imported raw materials, especially in situations of global shortage. The rising cost of raw material and a high level of quality assurance further complicate operations for manufacturers, while varied timelines for regulatory approvals of new vaccines can push back market entry and limit product availability.
Competitive Landscape:
The market in the Indian region is highly competitive, and many prominent local companies share the market by effectively producing vaccines at a lower cost. They are also increasing their capacities, research, and development in new vaccine technology like mRNA, intranasally administered vaccines, and catering to the segment markets of pediatrics, adults, and travel vaccines. Strategic collaborations, technology transfers, and public-private networks in international markets are increasing competition in the market and inculcating innovation. The market has opportunities in exporting vaccines to low and middle-class nations, competing in terms of efficient costing, regulatory structures, and capacity to handle the market demands in the domestic and international markets.
Some of the major market players are:
- GlaxoSmithKline
- Sanofi Aventis
- Serum Institute of India
- Panacea Biotec
- Pfizer
- Novartis
- VHB Lifesciences
- Zydus Cadila
- MSD
Recent Developments:
- September 2025: Zydus Lifesciences launched VaxiFlu, India's first trivalent influenza vaccine aligned with WHO's 2025-26 Northern Hemisphere flu season recommendations. The vaccine incorporates three WHO-recommended strains and marks India's transition to globally adopted trivalent flu protection, developed at the company's Vaccine Technology Centre in Ahmedabad.
- December 2024: Bavarian Nordic entered a license and manufacturing agreement with Serum Institute of India for the MVA-BN mpox vaccine. The technology transfer will enable supply for the Indian market while expanding global manufacturing capacity for equitable access during mpox outbreaks.
Indian Vaccine Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2025 |
| Historical Period | 2020-2025 |
| Forecast Period | 2026-2034 |
| Units | Million, Billion INR |
|
Scope of the Report
|
Exploration of Historical and Forecast Trends, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Monovalent and Combined Vaccines Covered | BCG, HIB, Influenza, Varicella, Typhoid, Japanese Encephalitis, Measles, Tetanus Toxoid, Hepatitis A, Rubella, Diphtheria, Tetanus, and Pertussis (DPT), Oral Polio Vaccine (OPV), MMR, Rotavirus, Hepatitis B, Pneumococcal, Meningococcal, Rabies, HPV, Hexavalent, Dengue vaccines |
| Companies Covered | GlaxoSmithKline, Sanofi Aventis, Serum Institute of India, Panacea Biotec, Pfizer, Novartis, VHB Lifesciences, Zydus Cadila and MSD. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report
The Indian vaccine market size was valued at USD 1.49 Billion in 2025.
The Indian vaccine market is expected to grow at a compound annual growth rate of 8.5% from 2026-2034 to reach USD 3.09 Billion by 2034.
Oral polio vaccine (OPV), representing the largest revenue share of 8% in 2025, remains pivotal for India's immunization landscape. The segment's dominance reflects sustained government commitment to polio elimination efforts and the Universal Immunization Programme's comprehensive coverage targeting millions of newborns annually.
Key factors driving the Indian vaccine market include extensive government immunization programs, rising awareness about preventive healthcare, robust domestic manufacturing capabilities, expanding healthcare infrastructure, and India's strategic role in global vaccine supply chains.
Major challenges include cold chain infrastructure limitations in rural areas, vaccine hesitancy driven by misinformation, regulatory compliance requirements, supply chain vulnerabilities for raw materials, and the need for continued investment in advanced vaccine technology platforms.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












