
India Gene Therapy Market Size, Share, Trends and Forecast by Gene Type, Vector Type, Delivery Method, Application, and Region, 2025-2033
Market Overview:
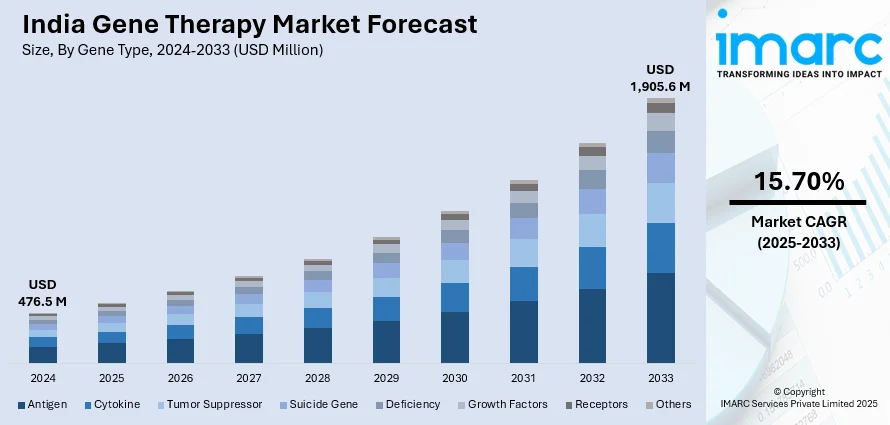
The India gene therapy market size reached USD 476.5 Million in 2024. Looking forward, IMARC Group expects the market to reach USD 1,905.6 Million by 2033, exhibiting a growth rate (CAGR) of 15.70% during 2025-2033. The growing awareness and acceptance of gene therapy among healthcare professionals and patients, rising prevalence of genetic disorders in India, the considerable rise in funding for gene therapy research and development (R&D) activities, and strategic collaborations between biotech firms, academic institutions, and healthcare providers represent some of the key factors driving the market.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 476.5 Million |
|
Market Forecast in 2033
|
USD 1,905.6 Million |
| Market Growth Rate 2025-2033 | 15.70% |
Gene therapy is a cutting-edge medical approach that holds immense promise in revolutionizing the treatment of genetic disorders and inherited diseases. It is a novel therapeutic strategy that involves the introduction, modification, or replacement of genes within a patient's cells to correct or compensate for faulty genetic information. Gene therapy is characterized by its potential for long-term and even a complete cure for certain disorders, tailored treatment procedures based on unique genetic profiles, enhanced efficacy and reduced side effects. The working mechanism of gene therapy revolves around introducing functional genes into a patient's cells to compensate for defective or non-functional genes. This can be achieved through several approaches, including gene augmentation (adding copies of functional genes), gene editing (modifying the existing genes), and gene silencing (blocking or inhibiting harmful genes). The treatment process typically begins with the isolation of healthy genes, which are then delivered into the patient's cells using various delivery mechanisms, such as viral vectors or non-viral methods. The introduced genes work alongside the patient's natural genetic material to produce necessary proteins and restore normal cellular functions.
To get more information of this market, Request Sample
India Gene Therapy Market Trends:
The market in India is primarily driven by the growing awareness and acceptance of gene therapy among healthcare professionals and patients. This can be attributed to the rising prevalence of genetic disorders in India leading to an urgent need for innovative therapeutic solutions. In line with this, the considerable rise in funding for gene therapy research and development (R&D) activities by public as well as private agencies are providing a impetus to the market. Moreover, strategic collaborations between biotech firms, academic institutions, and healthcare providers expediting gene therapy advancements are creating a positive market outlook. In addition to this, the improving healthcare infrastructure and access to advanced medical facilities are resulting in a higher adoption of gene therapy in India. Besides this, the easy availability of skilled and growing workforce of geneticists, biotechnologists, and molecular biologists is advancing gene therapy research and development, which in turn is stimulating the market growth. The market is further driven by the continual advancements in gene editing technologies, such as CRISPR-Cas9 and safer gene delivery systems resulting in enhanced precision and efficacy of gene therapy interventions. Apart from this, favorable government initiatives granting orphan drug designations to gene therapies for rare diseases along with healthcare reforms and policy changes prioritizing affordable treatments for genetic diseases are fueling the market. Some of the other factors contributing to the market include rapid expansion of gene therapy clinical trials in India, streamlined and scalable manufacturing processes for gene therapies, and numerous public awareness campaigns conducted by healthcare organizations and companies.
India Gene Therapy Market Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the India gene therapy market report, along with forecasts at the country levels for 2025-2033. Our report has categorized the market based on gene type, vector type, delivery method, and application.
Gene Type Insights:
- Antigen
- Cytokine
- Tumor Suppressor
- Suicide Gene
- Deficiency
- Growth Factors
- Receptors
- Others
The report has provided a detailed breakup and analysis of the market based on the gene type. This includes antigen, cytokine, tumor suppressor, suicide gene, deficiency, growth factors, receptors, and others.
Vector Type Insights:
- Viral Vector
- Adenoviruses
- Lentiviruses
- Retroviruses
- Adeno-Associated Virus
- Herpes Simplex Virus
- Poxvirus
- Vaccinia Virus
- Others
- Non-Viral Techniques
- Naked and Plasmid Vectors
- Gene Gun
- Electroporation
- Lipofection
- Others
The report has provided a detailed breakup and analysis of the market based on the vector type. This includes viral vector (adenoviruses, lentiviruses, retroviruses, adeno-associated virus, herpes simplex virus, poxvirus, vaccinia virus, and others) and non-viral techniques (naked and plasmid vectors, gene gun, electroporation, lipofection, and others).
Delivery Method Insights:
- In-Vivo Gene Therapy
- Ex-Vivo Gene Therapy
A detailed breakup and analysis of the market based on the delivery method has also been provided in the report. This includes in-vivo gene therapy and ex-vivo gene therapy.
Application Insights:
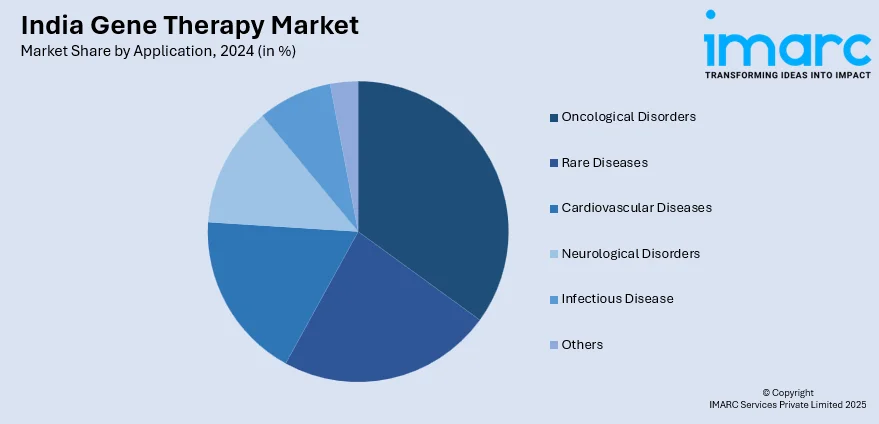
- Oncological Disorders
- Rare Diseases
- Cardiovascular Diseases
- Neurological Disorders
- Infectious Disease
- Others
A detailed breakup and analysis of the market based on the application has also been provided in the report. This includes oncological disorders, rare diseases, cardiovascular diseases, neurological disorders, infectious disease, and others.
Regional Insights:
- South India
- North India
- West & Central India
- East India
The report has also provided a comprehensive analysis of all the major regional markets, which include South India, North India, West & Central India, and East India.
Competitive Landscape:
The report has also provided a comprehensive analysis of the competitive landscape in the India gene therapy market. Competitive analysis such as market structure, key player positioning, top winning strategies, competitive dashboard, and company evaluation quadrant has been covered in the report. Also, detailed profiles of all major companies have been provided.
India Gene Therapy Market Report Coverage:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Million USD |
| Scope of the Report | Exploration of Historical and Forecast Trends, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Gene Types Covered | Antigen, Cytokine, Tumor Suppressor, Suicide Gene, Deficiency, Growth Factors, Receptors, Others |
| Vector Types Covered |
|
| Delivery Methods Covered | In-Vivo Gene Therapy, Ex-Vivo Gene Therapy |
| Applications Covered | Oncological Disorders, Rare Diseases, Cardiovascular Diseases, Neurological Disorders, Infectious Disease, Others |
| Regions Covered | South India, North India, West & Central India, South India |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Questions Answered in This Report:
- How has the India gene therapy market performed so far and how will it perform in the coming years?
- What is the breakup of the India gene therapy market on the basis of gene type?
- What is the breakup of the India gene therapy market on the basis of vector type?
- What is the breakup of the India gene therapy market on the basis of delivery method?
- What is the breakup of the India gene therapy market on the basis of application?
- What are the various stages in the value chain of the India gene therapy market?
- What are the key driving factors and challenges in the India gene therapy market?
- What is the structure of the India gene therapy market and who are the key players?
- What is the degree of competition in the India gene therapy market?
Key Benefits for Stakeholders:
- IMARC’s report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the India gene therapy market from 2019-2033.
- The research study provides the latest information on the market drivers, challenges, and opportunities in the India gene therapy market.
- Porter's five forces analysis assist stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the India gene therapy industry and its attractiveness.
- Competitive landscape allows stakeholders to understand their competitive environment and provides an insight into the current positions of key players in the market.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












