
Herpes Simplex Virus Treatment Market Size, Share, Trends and Forecast by Type, Drug Type, Route of Administration, Distribution Channel, and Region, 2025-2033
Herpes Simplex Virus Treatment Market Size and Share:
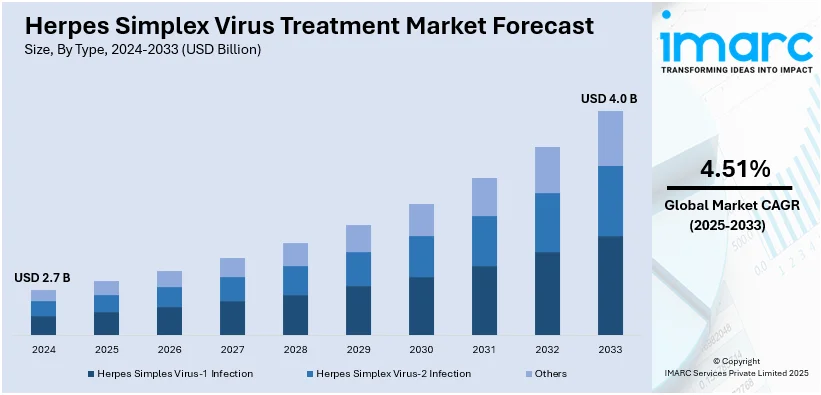
The global herpes simplex virus treatment market size was valued at USD 2.7 Billion in 2024. Looking forward, IMARC Group estimates the market to reach USD 4.0 Billion by 2033, exhibiting a CAGR of 4.51% during 2025-2033. North America currently dominates the market. The growing awareness among individuals about various medications of HSV, increasing healthcare expenditure, and wide availability of HSV medications through online and offline pharmacy stores represent some of the key factors driving the market.
|
Report Attribute
|
Key Statistics
|
|---|---|
|
Base Year
|
2024
|
|
Forecast Years
|
2025-2033
|
|
Historical Years
|
2019-2024
|
|
Market Size in 2024
|
USD 2.7 Billion |
|
Market Forecast in 2033
|
USD 4.0 Billion |
| Market Growth Rate 2025-2033 | 4.51% |
The global herpes simplex virus (HSV) treatment market is driven by a rising prevalence of HSV infections, increased awareness of sexually transmitted infections (STIs), and advancements in antiviral therapies. According to World Health Organization (WHO), about 3.8 billion individuals under the age of 50 (64%) worldwide are infected with herpes simplex virus type 1 (HSV-1), which is the main cause of oral herpes, and 520 million aged 15–49 (13%) are infected with herpes simplex virus type 2 (HSV-2), which is the main cause of genital herpes. Besides this, the growing demand for effective treatment options is further propelled by the availability of improved diagnostic tools and a stronger emphasis on early disease detection. Along with this, government initiatives promoting STI awareness and funding for research and development also play a crucial role in herpes simplex virus (HSV) treatment market growth. Additionally, considerable rise in public and private investments to develop innovative treatment methods, including vaccines and topical medications, is enhancing market growth. A shifting focus toward patient-centric care and expanding healthcare infrastructure in developing regions is creating a positive market outlook.
The United States stands out as a key regional market, primarily driven by an increasing burden of HSV-related complications, including neonatal herpes and genital herpes outbreaks, which demand advanced medical interventions. In line with this, the growing adoption of combination therapies and personalized medicine is enhancing treatment outcomes, further driving the market growth. Additionally, substantial investments by pharmaceutical companies in research and development (R&D) for targeted antiviral therapies is also expanding treatment options. On 19th November 2024, NOWDiagnostics, Inc., announced the release of the First To Know Syphilis Test, a novel FDA-authorized over-the-counter syphilis test in the U.S. With results as fast as 15 minutes based on a small blood drop, this elf-administered kit provides a confidential and convenient method for detecting sexually transmitted infections. It is available both online and at pharmacies nationwide, offering individuals a private and reliable alternative for managing their health. Moreover, continual technological advancements in drug delivery systems, such as nanotechnology-based solutions, are making treatments more effective and accessible. Concurrently, the presence of leading biopharmaceutical firms and a well-established healthcare system in the U.S. supports the rapid adoption of innovative products, bolstering the US herpes simplex virus (HSV) treatment market share.
Herpes Simplex Virus Treatment Market Trends:
Increasing Demand for Oral Antiviral Drugs
According to the U.S. Centers for Disease Control and Prevention (CDC), these drugs, such as oral antiviral drugs: Acyclovir and Valacyclovir, will remain the first-line treatments against HSV infections, especially recurrent cases of genital herpes. Oral antiviral therapies have cut HSV transmission rates by 70 to 80%. In 2024, close to 16% of the world's population aged between 15 and 49 years will be infected with HSV-2, thus creating a need for effective oral treatments. The more emphasis on declining transmission rates and better results for patients enhances the continued need for oral antiviral drugs. As HSV continues to multiply globally, so will the treatment demand for the same infections since they are the most reliable way of managing the condition.
Growing Use of Topical Antiviral Treatments
Topical antiviral preparations, such as topical creams and ointments, are employed for treating HSV recurrences in most cases. The CDC indicates that patients with topical treatments, which include medicines including Docosanol, experience an outbreak that could be shortened by 1 to 2 days if applied early in the course of an infection. According to the CDC, by 2023, over 50% of patients with HSV preferred topical treatment because it is easy and can be applied directly to the affected area. Ease of use and fast relief are factors that support the increasing popularity of these treatments. Furthermore, topical antivirals are recommended as adjunctive therapies to oral drugs to manage localized lesions.
Development of Vaccines for Herpes Simplex Virus
The development of herpes simplex virus vaccine is being researched, and the National Institutes of Health (NIH) are pushing to lower the rate of infections that are caused by herpes simplex virus. As of 2024, NIH have been funding several clinical studies that evaluate the efficacy of HSV vaccines. The global prevalence of HSV-2 infections is estimated at over 500 million cases. Thus, the urgent need for prevention solutions is important. Clinical studies by the NIH and other institutions have shown that an effective vaccine could significantly reduce the rates of HSV transmission and long-term treatment costs for those infected with the virus.
Herpes Simplex Virus Treatment Industry Segmentation:
IMARC Group provides an analysis of the key trends in each segment of the global herpes simplex virus treatment market, along with forecast at the global, regional, and country levels from 2025-2033. The market has been categorized based on type, drug type, route of administration and distribution channel.
Analysis by Type:
- Herpes Simples Virus-1 Infection
- Herpes Simplex Virus-2 Infection
- Others
Herpes Simplex Virus-1 (HSV-1) primarily causes oral herpes, characterized by cold sores around the mouth and face. The HSV-1 treatment market is driven by increasing awareness, improved diagnostic tools, and innovative antiviral therapies. Current treatment options include over-the-counter medications, prescription antiviral drugs such as acyclovir, and emerging topical treatments. Research into vaccine development and novel therapeutic strategies is further enhancing the potential for effective management of HSV-1 infections.
Herpes Simplex Virus-2 (HSV-2) mainly causes genital herpes, which is a lifetime disease with the episodic attacks. The focus of the treatment market for HSV-2 revolves around antiviral drugs like valacyclovir and famciclovir that suppress the activity of viruses and reduce its transmission. Greater public health interventions and education efforts have reduced stigma while promoting earlier diagnosis and treatment. Drug innovation and vaccine development will further improve the range of options for treating HSV-2 infections.
Analysis by Drug Type:
- Acyclovir
- Valacyclovir
- Famciclovir
- Others
Acyclovir leads the market in 2024 due to its proven efficacy, widespread availability, and affordability. As one of the first antiviral drugs developed specifically for herpes infections, acyclovir effectively manages outbreaks of both HSV-1 and HSV-2 by inhibiting viral replication. Its oral, topical, and intravenous formulations provide versatility, catering to various patient needs and severity levels. The drug’s established safety profile and minimal side effects further contribute to its dominance in the market. Additionally, generic versions of acyclovir ensure accessibility, particularly in cost-sensitive markets. With ongoing advancements in drug formulations, acyclovir continues to set the benchmark for effective antiviral therapies, reinforcing its stronghold in this competitive sector.
Analysis by Route of Administration:
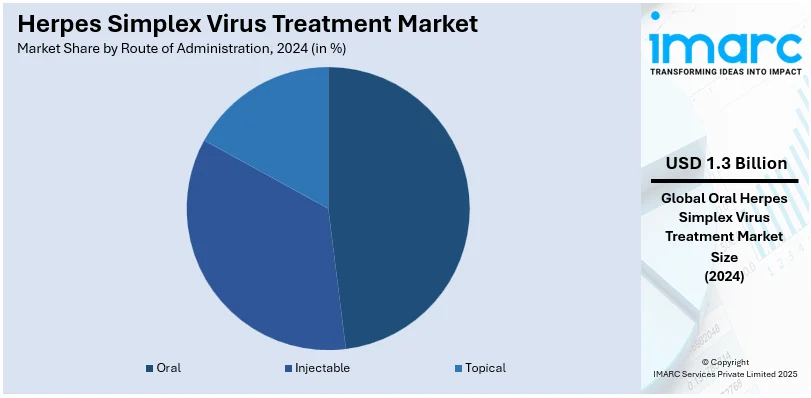
- Oral
- Injectable
- Topical
Oral leads the market in 2024 due to its convenience, efficacy, and widespread patient preference. Oral antiviral medications, such as acyclovir, valacyclovir, and famciclovir, provide effective systemic relief from both HSV-1 and HSV-2 infections, making them a cornerstone of treatment. This route simplifies dosing, enhancing patient compliance and accessibility compared to topical or intravenous alternatives. Oral formulations are particularly favored for managing recurrent outbreaks and for long-term suppressive therapy, offering sustained viral suppression and reduced transmission risk. With advancements in formulation technology, such as extended-release tablets, the oral route continues to strengthen its position as the most practical and widely used method for managing herpes simplex virus infections globally.
Analysis by Distribution Channel:
- Hospital Pharmacy
- Drug Stores
- Retail Stores
- Online Pharmacy
Hospital pharmacy leads the market in 2024 due to their pivotal role in providing access to prescription antiviral medications. These facilities ensure the availability of critical treatments including acyclovir and valacyclovir for acute and severe infections, often requiring immediate attention. Hospital pharmacies are integral to managing inpatient care, especially for intravenous drug formulations used in complex cases. Their stringent quality control measures, reliable supply chains, and expert pharmaceutical guidance enhance trust and patient outcomes. Additionally, they cater to a diverse patient demographic, from emergency cases to those requiring suppressive therapy. As healthcare infrastructure improves globally, hospital pharmacies continue to dominate as a trusted and indispensable distribution channel in the antiviral treatment market.
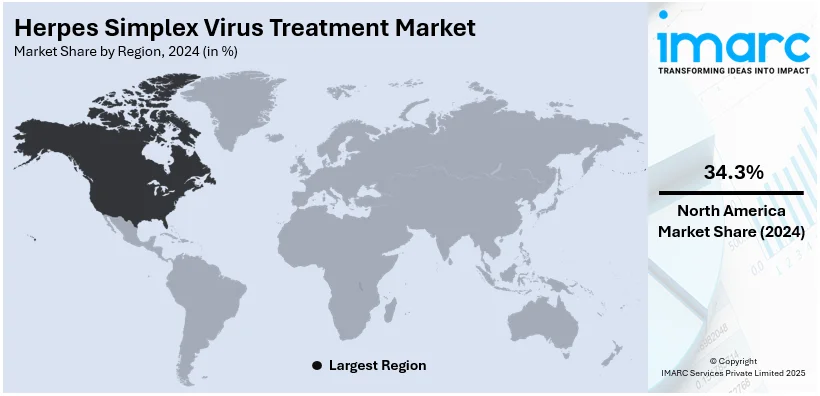
Regional Analysis:
- North America
- United States
- Canada
- Asia-Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
In 2024, North America accounted for the largest market share, driven by advanced healthcare infrastructure, high awareness levels, and significant healthcare expenditure. The region's well-established pharmaceutical sector is at the forefront of developing and distributing HSV treatments, which include antiviral medications and topical ointments. Additionally, North America benefits from a strong regulatory framework and widespread access to healthcare, facilitating prompt diagnosis and treatment. The growing prevalence of HSV infections, coupled with an aging population and the increased focus on sexually transmitted diseases (STDs), further enhances the demand for effective treatment options. This market dominance is expected to continue as research and innovation in antiviral therapies advance.
Key Regional Takeaways:
United States Herpes Simplex Virus Treatment Market Analysis
The U.S. herpes simplex virus (HSV) treatment market is growing steadily and is based on increasing knowledge of the virus and the need to have appropriate management solutions for it. According to the CDC (Center for Disease Control and Prevention), nearly 1 in 6 people aged 14-49 years was affected by genital herpes during 2023, thus there being a steady demand for antiviral treatments. The market reaps the benefit of a sound health care infrastructure and high levels of treatment adherence, including prescription antiviral drugs such as acyclovir as well as the over-the-counter varieties. High adoption levels of telemedicine and electronic prescriptions have streamlined access to treatments. Large players in the market are improving product lines by introducing new forms and combinations to enhance adherence among patients. The market is also progressing in terms of technology by the development of long-acting injectable therapies to offer more comfortable treatment choices.
Europe Herpes Simplex Virus Treatment Market Analysis
Rising prevalence rates and supportive health initiatives for the treatment of HSV in the European market have increased growth. A study states that close to 12% of Europe's adult population has chronic infection by HSV-2. The demand for antiviral therapies is constantly driven through such prevalence, while both oral and topical medicines remain highly prescribed with health systems emphasizing the better provision for these treatments. The emergence of resistant HSV strains has given birth to the development of new next-generation therapies, including vaccines and long-acting antivirals. The research is actively being carried on by pharmaceutical companies such as Gilead Sciences through clinical trials on innovative treatments. Moreover, public health campaigns reducing stigma and encouraging early diagnosis should improve treatment rates. Further to this, the adoption of digital health platforms for prescription services is further facilitating market growth, making treatments more accessible across the region.
Asia Pacific Herpes Simplex Virus Treatment Market Analysis
This treatment market for HSV in Asia Pacific is rapidly increasing due to higher awareness and increased access to healthcare in developing economies. According to WHO, in the Asia Pacific region, the prevalence of HSV-1 is approximately 50%, though there has been a sudden increase in cases of HSV-2 caused by new sexual behaviors. Antiviral treatments have become more accessible due to increasing adoption of telehealth platforms and e-prescriptions in India and China. Pharmaceutical investment in the region is also creating a market for this area, with companies such as Roche and Abbott expanding their portfolios in antivirals. Innovative treatment options including long-acting injectable antivirals are also gaining traction. Further, growth of healthcare infrastructure and increased disposable income will continue to enhance the growth of the market in the coming years, mainly in Southeast Asia where there is growing demand for effective therapies.
Latin America Herpes Simplex Virus Treatment Market Analysis
High infection rates and increased awareness of antiviral therapies drive the Latin American HSV treatment market. According to an industrial report, seroprevalence of herpes simplex virus type 1 in the region is high, with a pooled mean of 83.1%: 57.2% in children, 84.5% in healthy adults, and 90.9% in clinical adults. Such a high prevalence demands effective treatments such as acyclovir and valacyclovir. Public health campaigns target increased availability of antiviral treatments, particularly to underdeveloped communities. Companies such as Teva and Sanofi are improving market presence by localizing the manufacturing and distribution bases. Moreover, increasing disposable income and enhanced healthcare insurance are helping to enhance market performance. Greater awareness of healthy sexuality and protection measures are also augmenting the use of advanced therapies across the region.
Middle East and Africa Herpes Simplex Virus Treatment Market Analysis
The Middle East and Africa region is characterized by high infection rates and growing awareness in health care. A study has revealed that 88.8% of the population in Saudi Arabia is infected with HSV-1, mostly contracted before adulthood. The higher prevalence leads to constant demand for antiviral treatments, especially for symptomatic and recurrent cases. Regional governments have been investing in healthcare infrastructures to enhance access to treatment, including oral and topical antivirals. International and local pharmaceutical firms collaborate for the better availability of the effective therapies in the region. Public health campaigns towards educating the population on how to prevent and manage HSV are also contributing to growth in this market. However, it's challenging in rural areas to access health care, which calls for mobile health initiatives and telemedicine solutions.
Competitive Landscape:
The competitive landscape of the herpes simplex virus treatment market is influenced by the presence of key pharmaceutical companies focusing on the development and commercialization of innovative antiviral therapies. These companies are heavily investing in research and development to introduce more effective and targeted treatments, aiming to enhance patient outcomes while reducing side effects. Apart from conventional antiviral drugs, gene therapies and immune-modulating drugs are being developed by players. Companies are making strategic partnerships, mergers, and acquisitions in order to broaden product portfolios and expand market reach. Such companies are upgrading their marketing strategies, their distribution networks, and focusing on personalized medicine to address the diverse needs of patients worldwide.
The report provides a comprehensive analysis of the competitive landscape in the herpes simplex virus treatment market with detailed profiles of all major companies, including:
- Agenus Inc.
- Apotex Inc.
- Avet Pharmaceuticals Inc.
- Carlsbad Tech
- EPI Health LLC
- F. Hoffmann-La Roche Ltd
- Fresenius SE & Co. KGaA
- GlaxoSmithKline plc
- Glenmark Pharmaceuticals Limited
- Merck & Co. Inc.
- Novartis AG
- Sanofi S.A.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
Latest News and Developments:
- September 2024: GSK reported that part II of the TH HSV REC-003 trial regarding its HSV vaccine candidate GSK3943104 fails the primary objective for its efficacy and will not move to phase III. No safety concerns were highlighted. GSK will, however, continue monitoring safety and generating follow-up data to gain recurrent genital herpes insights.
- July 2024: Preclinical data for investigational helicase-primase inhibitors ABI-5366 and ABI-1179 were presented by Assembly Biosciences at the International Herpesvirus Workshop on July 15, 2024. ABI-5366 data will support entry into Phase 1a/b clinical trials, while ABI-1179 is expected to enter the clinic by the end of 2024.
Herpes Simplex Virus Treatment Market Report Scope:
| Report Features | Details |
|---|---|
| Base Year of the Analysis | 2024 |
| Historical Period | 2019-2024 |
| Forecast Period | 2025-2033 |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Types Covered | Herpes Simples Virus-1 Infection, Herpes Simplex Virus-2 Infection, Others |
| Drug Types Covered | Acyclovir, Valacyclovir, Famciclovir, Others |
| Route of Administrations Covered | Oral, Injectable, Topical |
| Distribution Channels Covered | Hospital Pharmacy, Drug Stores, Retail Stores, Online Pharmacy |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Agenus Inc., Apotex Inc., Avet Pharmaceuticals Inc., Carlsbad Tech, EPI Health LLC, F. Hoffmann-La Roche Ltd, Fresenius SE & Co. KGaA, GlaxoSmithKline plc, Glenmark Pharmaceuticals Limited, Merck & Co. Inc., Novartis AG, Sanofi S.A., Teva Pharmaceutical Industries Ltd., Viatris Inc., etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Key Benefits for Stakeholders:
- IMARC’s industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the herpes simplex virus treatment market from 2019-2033.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the global herpes simplex virus treatment market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the herpes simplex virus treatment industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
Key Questions Answered in This Report
The herpes simplex virus treatment market was valued at USD 2.7 Billion in 2024.
The market is estimated to reach USD 4.0 Billion by 2033, exhibiting a CAGR of 4.51% during 2025-2033.
Key drivers include rising HSV infection prevalence, increased awareness of STIs, advancements in antiviral therapies, and greater access to healthcare. Government initiatives and research funding are also crucial in promoting effective treatment options.
North America currently dominates the herpes simplex virus treatment market. The growing awareness among individuals about various medications of HSV, increasing healthcare expenditure, and wide availability of HSV medications through online and offline pharmacy stores represent some of the key factors driving the regional market.
Some of the major players in the global herpes simplex virus treatment market include Agenus Inc., Apotex Inc., Avet Pharmaceuticals Inc., Carlsbad Tech, EPI Health LLC, F. Hoffmann-La Roche Ltd, Fresenius SE & Co. KGaA, GlaxoSmithKline plc, Glenmark Pharmaceuticals Limited, Merck & Co. Inc., Novartis AG, Sanofi S.A., Teva Pharmaceutical Industries Ltd. and Viatris Inc., among others.
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Request Customization
Request Customization
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Inquire Before Buying
Inquire Before Buying




.webp)




.webp)












