
Heart Failure Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035
Market Overview:
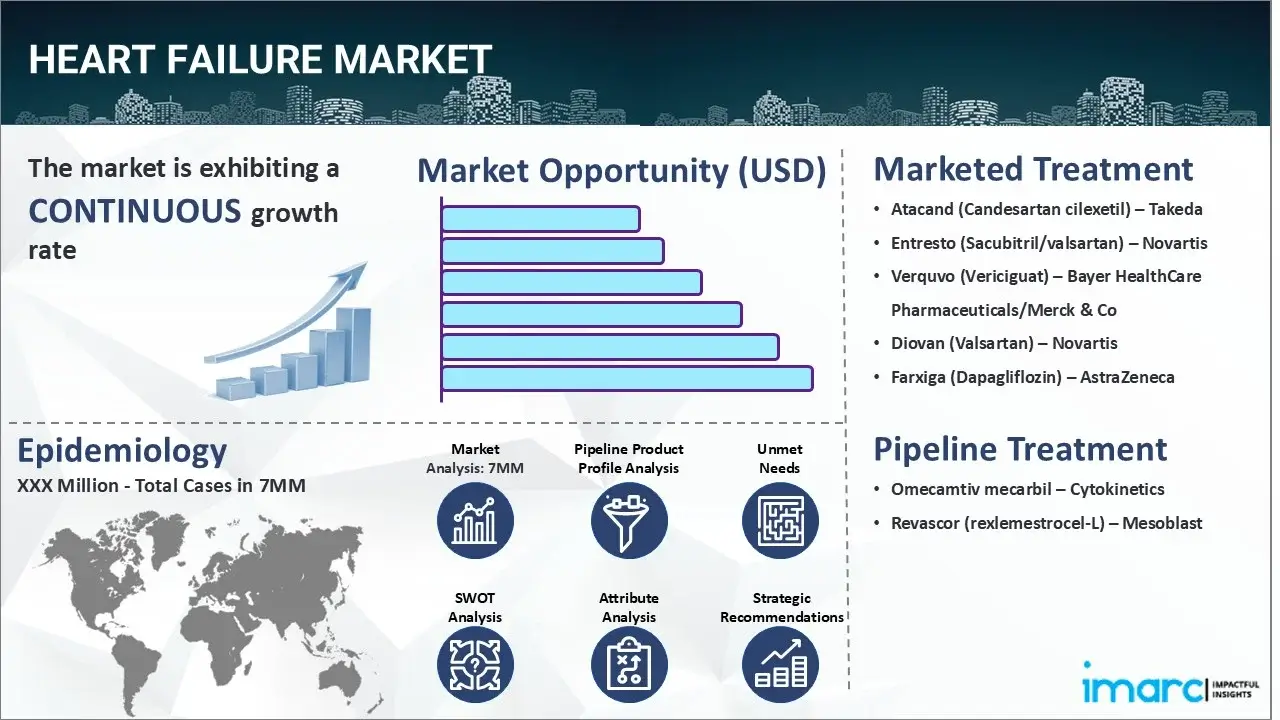
The heart failure market reached a value of USD 7.3 Billion across the top 7 markets (US, EU4, UK, and Japan) in 2024. Looking forward, IMARC Group expects the top 7 major markets to reach USD 19.5 Billion by 2035, exhibiting a growth rate (CAGR) of 9.42% during 2025-2035.
|
Report Attribute
|
Key Statistics
|
|---|---|
| Base Year |
2024
|
| Forecast Years | 2025-2035 |
| Historical Years |
2019-2024
|
|
Market Size in 2024
|
USD 7.3 Billion |
|
Market Forecast in 2035
|
USD 19.5 Billion |
|
Market Growth Rate 2025-2035
|
9.42% |
The heart failure market has been comprehensively analyzed in IMARC's new report titled "Heart Failure Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook". Heart failure, also referred to as congestive heart failure, is a chronic, progressive condition in which the heart cannot pump enough blood to meet the body's requirements. This can happen due to a variety of factors, such as high blood pressure, damage to the heart muscle, or a previous heart attack. Heart failure is classified into two types: heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF). While HFpEF results from a stiff left ventricle that cannot fill with blood appropriately, HFrEF happens when the heart's left ventricle is unable to pump blood efficiently. Some of the symptoms of heart failure include shortness of breath, swelling in the legs and feet, fatigue, and rapid or irregular heartbeat. An electrocardiogram (ECG), echocardiography, chest x-ray, and blood tests may be performed to identify heart failure. The treatment typically includes a combination of lifestyle changes, such as adopting a heart-healthy diet and engaging in regular exercise, as well as medication to improve the heart's ability to pump blood. In some cases, surgery, including a heart transplant or a procedure to implant a heart pump, may be required.
To get more information on this market, Request Sample
The expanding geriatric population, who are more susceptible to diabetes and chronic cardiovascular diseases, is primarily driving the global heart failure market. In addition to this, the increasing prevalence of heart problems among the masses owing to obesity and lack of physical activities is also bolstering the market growth. Moreover, the rising incidences of several associated risk factors, such as excessive smoking and drinking of alcoholic beverages, along with the unhealthy dietary habits of individuals, are propelling the occurrence of heart problems, which is further catalyzing the global market. Apart from this, the emerging popularity of angiotensin-converting-enzyme (ACE) inhibitors, which work by relaxing the blood vessels and allowing the heart to pump blood more easily, is also creating a positive outlook for the market. Additionally, the widespread adoption of diuretics to remove excess fluid from the body, which can help to reduce heart failure symptoms, is further augmenting the global market. Besides this, several key players are making significant investments in introducing new drugs, such as sacubitril/valsartan, to lower the risk of cardiovascular mortality and heart failure in adult patients. This, in turn, is also acting as a significant growth-inducing factor. Furthermore, the increasing utilization of left ventricular assist device (LVAD) as a bridge to transplantation or as a destination therapy for patients who are not candidates for transplantation is expected to drive the global heart failure market in the coming years.
IMARC Group's new report provides an exhaustive analysis of the heart failure market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for heart failure and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the heart failure market in any manner.
Recent Developments:
- In April 2025, Mesoblast announced an update on its plans to meet with the U.S. FDA to discuss the accelerated approval pathway for Revascor (rexlemestrocel-L) in the treatment of patients with ischemic chronic heart failure with reduced ejection fraction and inflammation.
- In December 2024, Cytokinetics disclosed that COMET-HF (Confirmation of Omecamtiv Mecarbil Efficacy Trial in Heart Failure), a confirmatory Phase 3 clinical trial of omecamtiv mecarbil in patients with symptomatic heart failure with severely reduced ejection fraction, is open to enrollment.
- In May 2023, AstraZeneca announced that Farxiga (dapagliflozin) had been approved in the U.S. to reduce the risk of cardiovascular death, hospitalisation for heart failure and urgent heart failure (HF) visits in adults with HF. The approval by the FDA was based on positive results from the DELIVER Phase III trial.
Drugs:
Atacand (candesartan cilexetil) is a type of angiotensin II receptor blocker (ARB) used to treat heart failure (NYHA class II-IV) in individuals with left ventricular systolic dysfunction (ejection fraction ≤ 40%). It is intended to prevent cardiovascular deaths and hospitalizations. It inhibits the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking its binding to the AT1 receptor in various tissues, including vascular smooth muscle and the adrenal gland.
Omecamtiv mecarbil is a potent small-molecule cardiac myosin activator. This drug is designed to specifically target the contractile process, or pumping activity, of the heart. By activating cardiac myosin, a protein that converts chemical energy into a mechanical force that causes the heart to contract, omecamtiv mecarbil may enhance cardiac muscle performance. Preclinical studies have revealed that cardiac myosin activators boost cardiac contractility without changing the concentrations of intracellular myocyte calcium or myocardial oxygen consumption.
Revascor (rexlemestrocel-L) is an allogeneic mesenchymal precursor cell (MPC) therapy designed to treat heart failure by promoting cardiac repair and reducing inflammation. The MPCs secrete paracrine factors that modulate immune responses, reduce fibrosis, and stimulate endogenous repair pathways. This leads to improved myocardial function, reduced ventricular remodeling, and enhanced tissue regeneration, ultimately aiming to improve cardiac output and clinical outcomes in patients with chronic heart failure.
Time Period of the Study
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the heart failure market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the heart failure market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report also provides a detailed analysis of the current heart failure marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
| Drugs | Company Name |
|---|---|
| Atacand (Candesartan cilexetil) | Takeda |
| Entresto (Sacubitril/valsartan) | Novartis |
| Verquvo (Vericiguat) | Bayer HealthCare Pharmaceuticals/Merck & Co |
| Diovan (Valsartan) | Novartis |
| Farxiga (Dapagliflozin) | AstraZeneca |
| Jardiance (Empagliflozin) | Boehringer Ingelheim/Eli Lilly and Company |
| Invokana (canagliflozin) | Janssen Pharmaceuticals |
| Omecamtiv Mecarbil | Cytokinetics |
| Revascor (rexlemestrocel-L) | Mesoblast |
*Kindly note that the drugs in the above table only represent a partial list of marketed/pipeline drugs, and the complete list has been provided in the report.
Key Questions Answered in this Report:
Market Insights
- How has the heart failure market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2024 and how are they expected to perform till 2035?
- What was the country-wise size of the heart failure across the seven major markets in 2024 and what will it look like in 2035?
- What is the growth rate of the heart failure across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2019-2035) of heart failure across the seven major markets?
- What is the number of prevalent cases (2019-2035) of heart failure by age across the seven major markets?
- What is the number of prevalent cases (2019-2035) of heart failure by gender across the seven major markets?
- What is the number of prevalent cases (2019-2035) of heart failure by type across the seven major markets?
- How many patients are diagnosed (2019-2035) with heart failure across the seven major markets?
- What is the size of the heart failure patient pool (2019-2024) across the seven major markets?
- What would be the forecasted patient pool (2025-2035) across the seven major markets?
- What are the key factors driving the epidemiological trend of heart failure?
- What will be the growth rate of patients across the seven major markets?
Heart Failure: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for heart failure drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the heart failure market?
- What are the key regulatory events related to the heart failure market?
- What is the structure of clinical trial landscape by status related to the heart failure market?
- What is the structure of clinical trial landscape by phase related to the heart failure market?
- What is the structure of clinical trial landscape by route of administration related to the heart failure market?
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization




.webp)




.webp)












