
Chronic Hepatitis B Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035
Market Overview:
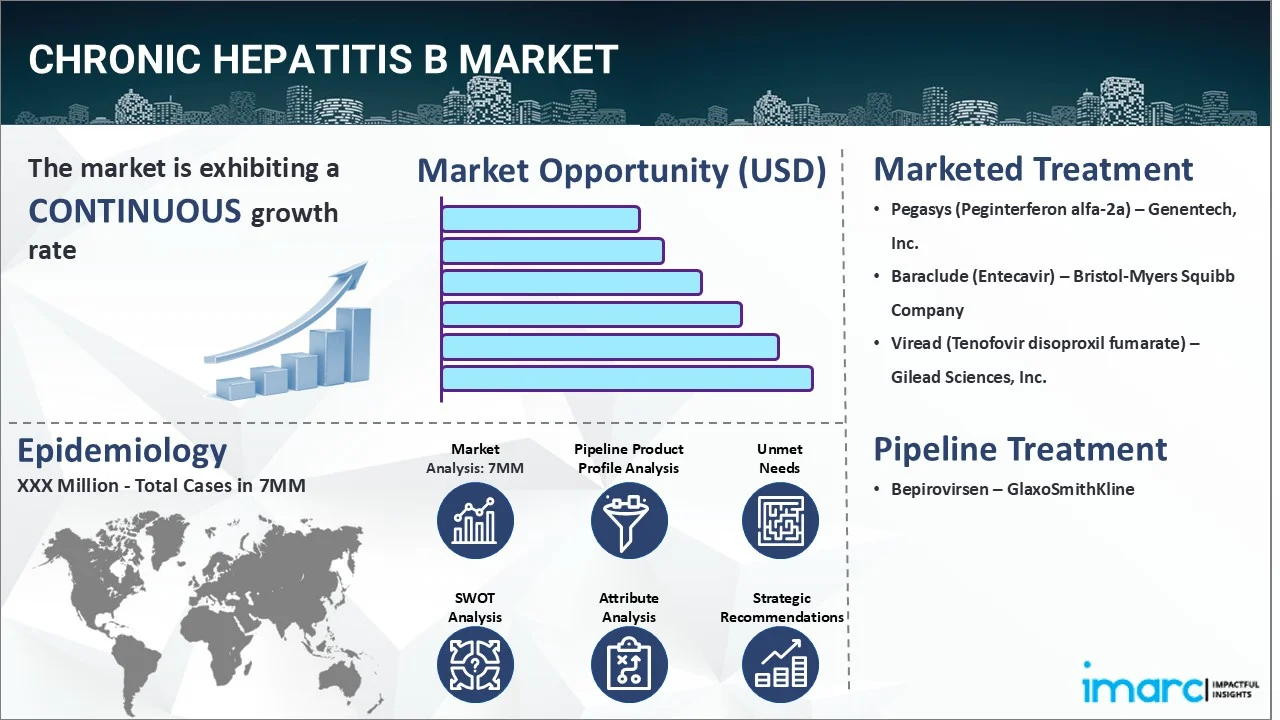
The chronic hepatitis B market reached a value of USD 2,764.4 Million across the top 7 markets (US, EU4, UK, and Japan) in 2024. Looking forward, IMARC Group expects the top 7 major markets to reach USD 3,482.3 Million by 2035, exhibiting a growth rate (CAGR) of 2.13% during 2025-2035.
|
Report Attribute
|
Key Statistics
|
|---|---|
| Base Year | 2024 |
| Forecast Years | 2025-2035 |
| Historical Years |
2019-2024
|
|
Market Size in 2024
|
USD 2,764.4 Million
|
|
Market Forecast in 2035
|
USD 3,482.3 Million
|
|
Market Growth Rate 2025-2035
|
2.13% |
The chronic hepatitis B market has been comprehensively analyzed in IMARC's new report titled "Chronic Hepatitis B Market Size, Epidemiology, In-Market Drugs Sales, Pipeline Therapies, and Regional Outlook 2025-2035". Chronic hepatitis B refers to a long-term viral infection caused by the hepatitis B virus (HBV) that affects the liver. It can manifest with a range of symptoms, although many individuals suffering from the ailment may remain asymptomatic for a long time. When indications do occur, they may include fatigue, nausea, abdominal discomfort, loss of appetite, jaundice (yellowing of the skin and eyes), etc. In some cases, the condition can progress to more advanced liver disease, leading to symptoms like fluid retention, easy bruising or bleeding, confusion, etc. The diagnosis of chronic hepatitis B involves a combination of blood tests and clinical evaluation. Serological tests are used to detect specific markers of the hepatitis B virus (HBV) infection, including hepatitis B e antigen (HBeAg), hepatitis B surface antigen (HBsAg), and antibodies against these antigens (anti-HBs and anti-HBe). HBV DNA testing is performed to measure the viral load in the blood. Additionally, various imaging studies, such as ultrasound and liver biopsy, may be conducted to evaluate liver structure and assess the degree of liver damage.
To get more information on this market, Request Sample
The increasing cases of unprotected sexual intercourse with an infected partner, sharing needles or other drug paraphernalia, coming into contact with infected blood or body fluids, etc., are primarily driving the chronic hepatitis B market. In addition to this, the widespread adoption of numerous antiviral agents, such as nucleotide/nucleoside analogs and pegylated interferon-alpha (PEG-IFN), for suppressing viral replication, decreasing liver inflammation, and slowing down the progression of the disease is also creating a positive outlook for the market. Moreover, the inflating application of liver fibrosis assessment methods, including transient elastography and serum biomarkers, since they offer a safer and less invasive alternative to liver biopsy, thereby reducing patient discomfort as well as risks associated with invasive procedures, is acting as another significant growth-inducing factor. Apart from this, the emerging popularity of immune modulation strategies, which stimulate the immune system while leading to sustained viral suppression and even serological response, such as hepatitis B surface antigen (HBsAg) loss, is expected to drive the chronic hepatitis B market during the forecast period.
IMARC Group's new report provides an exhaustive analysis of the chronic hepatitis B market in the United States, EU4 (Germany, Spain, Italy, and France), United Kingdom, and Japan. This includes treatment practices, in-market, and pipeline drugs, share of individual therapies, market performance across the seven major markets, market performance of key companies and their drugs, etc. The report also provides the current and future patient pool across the seven major markets. According to the report, the United States has the largest patient pool for chronic hepatitis B and also represents the largest market for its treatment. Furthermore, the current treatment practice/algorithm, market drivers, challenges, opportunities, reimbursement scenario, unmet medical needs, etc., have also been provided in the report. This report is a must-read for manufacturers, investors, business strategists, researchers, consultants, and all those who have any kind of stake or are planning to foray into the chronic hepatitis B market in any manner.
Recent Developments:
- In February 2024, GlaxoSmithKline stated that the U.S. Food and Drug Administration (FDA) had given Fast Track designation for bepirovirsen, an experimental antisense oligonucleotide (ASO) for the treatment of chronic hepatitis B.
- In June 2023, Arbutus Biopharma Corporation announced that preliminary data from its ongoing Phase 2a clinical trial evaluating the safety, tolerability, and antiviral activity of the combination of AB-729, the Company's lead RNAi therapeutic, and pegylated interferon alfa-2a (IFN) in patients with chronic hepatitis B virus were presented at the European Association for the Study of the Liver (EASL) Congress. The preliminary data indicate that adding IFN to AB-729 medication was usually well tolerated and appears to result in ongoing HBsAg decreases in certain patients.
Key Highlights
- The World Health Organization (WHO) estimates that 254 million people are living with chronic hepatitis B, with 1.2 million new infections each year.
- Over 350 million people are chronic carriers of the hepatitis B virus.
- Approximately 75% of chronic carriers reside in Asia and the Western Pacific.
- It was reported that 15-40% of hepatitis B virus-infected patients would develop cirrhosis, liver failure, or HCC.
- Around 500,000 to 1.2 million persons die of hepatitis B virus infection each year.
Drugs:
Viread (Tenofovir disoproxil fumarate) is used to treat chronic hepatitis B virus in adults and children aged 2 and above who weigh at least 10 kg. For adults and pediatric patients 2 years and older weighing at least 17 kg, the recommended dosage of VIREAD tablet is 8 mg of tenofovir disoproxil fumarate (TDF) per kg of body weight (up to 300 mg) once daily.
Time Period of the Study
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the chronic hepatitis B market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the chronic hepatitis B market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report also provides a detailed analysis of the current chronic hepatitis B marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
| Drugs | Company Name |
|---|---|
| Pegasys (Peginterferon alfa-2a) | Genentech, Inc. |
| Baraclude (Entecavir) | Bristol-Myers Squibb Company |
| Viread (Tenofovir disoproxil fumarate) | Gilead Sciences, Inc. |
| Bepirovirsen | GlaxoSmithKline |
*Kindly note that the drugs in the above table only represent a partial list of marketed/pipeline drugs, and the complete list has been provided in the report.
Key Questions Answered in this Report:
Market Insights
- How has the Chronic Hepatitis B market performed so far and how will it perform in the coming years?
- What are the markets shares of various therapeutic segments in 2024 and how are they expected to perform till 2035?
- What was the country-wise size of the Chronic Hepatitis B across the seven major markets in 2024 and what will it look like in 2035?
- What is the growth rate of the Chronic Hepatitis B across the seven major markets and what will be the expected growth over the next ten years?
- What are the key unmet needs in the market?
Epidemiology Insights
- What is the number of prevalent cases (2019-2035) of Chronic Hepatitis B across the seven major markets?
- What is the number of prevalent cases (2019-2035) of Chronic Hepatitis B by age across the seven major markets?
- What is the number of prevalent cases (2019-2035) of Chronic Hepatitis B by gender across the seven major markets?
- How many patients are diagnosed (2019-2035) with Chronic Hepatitis B across the seven major markets?
- What is the size of the Chronic Hepatitis B’ patient pool (2019-2024) across the seven major markets?
- What would be the forecasted patient pool (2025-2035) across the seven major markets?
- What are the key factors driving the epidemiological trend of Chronic Hepatitis B?
- What will be the growth rate of patients across the seven major markets?
Chronic Hepatitis B: Current Treatment Scenario, Marketed Drugs and Emerging Therapies
- What are the current marketed drugs and what are their market performance?
- What are the key pipeline drugs and how are they expected to perform in the coming years?
- How safe are the current marketed drugs and what are their efficacies?
- How safe are the late-stage pipeline drugs and what are their efficacies?
- What are the current treatment guidelines for Chronic Hepatitis B drugs across the seven major markets?
- Who are the key companies in the market and what are their market shares?
- What are the key mergers and acquisitions, licensing activities, collaborations, etc. related to the Chronic Hepatitis B market?
- What are the key regulatory events related to the Chronic Hepatitis B market?
- What is the structure of clinical trial landscape by status related to the Chronic Hepatitis B market?
- What is the structure of clinical trial landscape by phase related to the Chronic Hepatitis B market?
- What is the structure of clinical trial landscape by route of administration related to the Chronic Hepatitis B market?
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.
 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure
 Request Customization
Request Customization




.webp)




.webp)












