Global Single-Use Medical Device Reprocessing Market Expected to Reach USD 2,533.6 Million by 2033 - IMARC Group
Global Single-Use Medical Device Reprocessing Market Statistics, Outlook and Regional Analysis 2025-2033
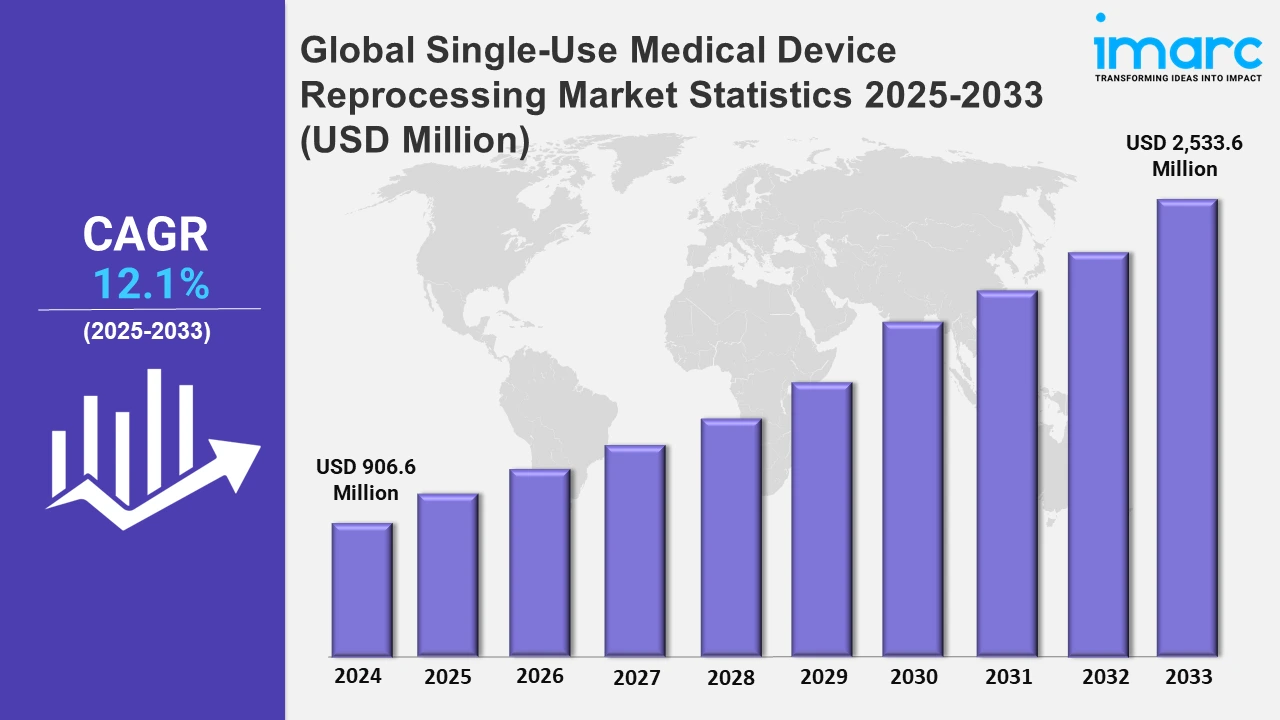
The global single-use medical device reprocessing market size was valued at USD 906.6 Million in 2024, and it is expected to reach USD 2,533.6 Million by 2033, exhibiting a growth rate (CAGR) of 12.1% from 2025 to 2033.
To get more information on this market, Request Sample
Hospitals and healthcare facilities are increasingly adopting single-use medical device reprocessing practices due to the potential for cost savings and reduced medical waste. Apart from this, reprocessing can lead to significant savings for medical facilities, compared to purchasing new devices. Environmental concerns are also propelling more facilities to reconsider their approach, aiming to lower their carbon footprint by minimizing medical waste. Innovative solutions are emerging within the reprocessing market. These launches incorporate advanced technology, such as enhanced sterilants and sophisticated monitoring systems, ensuring that reprocessed devices meet quality benchmarks akin to new equipment. In 2024, Innovative Health, Inc. entered into an agreement with MC healthcare, Inc. that will enable hospitals with MC Healthcare’s Japanese network to reduce environmental impact and at the same time allow Innovative Health’s US hospital partners to enhance their savings from single-use medical device reprocessing.
Regulatory backing is also playing a pivotal role in supporting the market growth. Authorities in various countries are laying out stringent guidelines for single-use medical device reprocessing to ensure safety and efficacy. This regulatory framework is boosting confidence in the safety of reprocessed devices. Devices that are commonly reprocessed include electrophysiology catheters, pulse oximeter sensors, and compression sleeves. Moreover, there is a notable increase in the reprocessing of more complex devices as technologies improved. The expanded categories are adding versatility to the market and allowed healthcare providers more options in reducing overall costs. The rise of automation in device reprocessing is bolstering the market growth. Automated systems optimize workflows, minimize human mistakes, and guarantee uniform sterilization. Advancements in artificial intelligence (AI) and the integration of the internet of things (IoT) in reprocessing machinery are resulting in enhanced tracking and data management, enabling improved documentation of reprocessing cycles and adherence to compliance.
Global Single-Use Medical Device Reprocessing Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share on account of the availability of advanced technologies and increasing focus on improving safety of reprocessing.
North America Single-Use Medical Device Reprocessing Market Trends:
The North American market is driven by cost-reduction strategies and sustainability initiatives. Hospitals and healthcare systems are increasingly adopting reprocessing practices to lower expenses, with savings compared to buying new devices. Besides this, advanced technologies and automated systems are enhancing the safety and efficiency of reprocessing. Regulatory backing, particularly by the U.S. FDA, provides a secure framework for this practice, boosting confidence in reprocessed devices. For instance, in 2024, the US FDA introduced online resources to present information about reprocessing single-use medical devices for healthcare facilities and frequently asked questions (FAQs). Apart from this, North America maintains its leading market position due to high healthcare expenditures and established infrastructure supporting sustainable practices.
Asia-Pacific Single-Use Medical Device Reprocessing Market Trends:
The Asia-Pacific region is rapidly advancing in the reprocessing market, driven by increasing healthcare investments and cost-saving initiatives in countries like India and China. Expanding healthcare infrastructure and a need for budget-friendly solutions are propelling the market growth. Regulatory adaptation is improving, with a focus on ensuring safety and efficacy.
Europe Single-Use Medical Device Reprocessing Market Trends:
Europe shows strong growth in the reprocessing market, fueled by stringent environmental regulations and a push for sustainable healthcare solutions in various healthcare facilities. Countries like Germany and France are spearheading the adoption, leveraging advanced reprocessing technologies to meet both cost and eco-friendly goals.
Latin America Single-Use Medical Device Reprocessing Market Trends:
In Latin America, the market is growing steadily, supported by cost-containment strategies and healthcare budget constraints. Brazil and Mexico are leading the regional uptake, driven by the need for cost-effective solutions amidst the growing medical needs and evolving healthcare policies in the region.
Middle East and Africa Single-Use Medical Device Reprocessing Market Trends:
The Middle East and Africa show promising growth potential in the reprocessing market. This trend is primarily driven by increasing healthcare initiatives and efforts to adopt sustainable practices. While the infrastructure is still developing, investments and partnerships are paving the way for expansion.
Top Companies Leading in the Single-Use Medical Device Reprocessing Industry
Some of the leading Single-Use Medical Device Reprocessing market companies include Arjo Inc., Innovative Health, Johnson & Johnson, Medline Industries LP, NEScientific Inc., Steripro Canada, Stryker Corporation, SureTek Medical, and Vanguard AG, among many others. In 2024, Arjo Inc. announced the opening of its new state-of-the-art facility for reprocessing medical devices. This new project is 50% larger than the previous location and is designed to increase the manufacturing output and majorly improve turnaround times.
Global Single-Use Medical Device Reprocessing Market Segmentation Coverage
- On the basis of the device type, the market has been bifurcated into class I devices (laparoscopic graspers, scalpels, tourniquet cuffs, and other class I devices) and class II devices (pulse oximeter sensors, sequential compression sleeves, catheters and guidewires, and other class II devices), wherein and class II devices (pulse oximeter sensors, sequential compression sleeves, catheters and guidewires, and other class II devices) represent the leading segment. Class II medical devices, including pulse oximeter sensors, sequential compression sleeves, catheters, and guidewires, play a significant role in the single-use medical device reprocessing market. These devices require stringent reprocessing protocols to ensure patient safety and efficacy. Advanced sterilization technologies and regulatory guidelines help ensure that reprocessed Class II devices meet safety criteria similar to new products.
- Based on the application, the market is classified into general surgery, anesthesia, arthroscopy and orthopedic surgery, cardiology, gastroenterology, gynecology, urology, and others. Reprocessed devices in general surgery help reduce operational costs while maintaining safety standards. Single-use devices like catheters and sensors used in anesthesia are increasingly reprocessed to support cost-efficient care. The use of reprocessed surgical tools in arthroscopy aids in lowering costs and waste in orthopedic procedures. Cardiology applications benefit from the reprocessing of catheters and guidewires, enhancing cost management in complex procedures. Reprocessed endoscopic tools support more affordable and sustainable practices in gastroenterology. Single-use devices in gynecology are reprocessed to optimize expenditures without compromising patient safety. Reprocessing in urology includes catheters, reducing expenses while ensuring effective treatment.
- On the basis of the end user, the market has been divided into hospitals, ambulatory surgical centers, and others. Among these, hospitals account for the majority of the market share. Hospitals represent the largest segment driven by the need to reduce operational costs and promote sustainability. Reprocessing enables hospitals to maintain high-quality patient care while cutting expenses by reusing devices like catheters, oximeter sensors, and surgical tools. Additionally, reprocessing aligns with environmental goals of hospitals by minimizing medical waste. Hospitals benefit from advancements in reprocessing technology and regulatory support, ensuring that reprocessed devices meet strict safety and performance standards.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 906.6 Million |
| Market Forecast in 2033 | USD 2,533.6 Million |
| Market Growth Rate 2025-2033 | 12.1% |
| Units | Million USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Device Types Covered |
|
| Applications Covered | General Surgery, Anesthesia, Arthroscopy and Orthopaedic Surgery, Cardiology, Gastroenterology, Gynaecology, Urology, Others |
| End Users Covered | Hospitals, Ambulatory Surgical Centers, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Arjo Inc., Innovative Health, Johnson & Johnson, Medline Industries LP, NEScientific Inc., Steripro Canada, Stryker Corporation, SureTek Medical, Vanguard AG etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.





.webp)




.webp)












