Sickle Cell Disease Market Size to Reach USD 2,011.9 Million by 2035, Impelled by Advancements in Early Detection
Sickle Cell Disease Market Outlook 2025-2035:
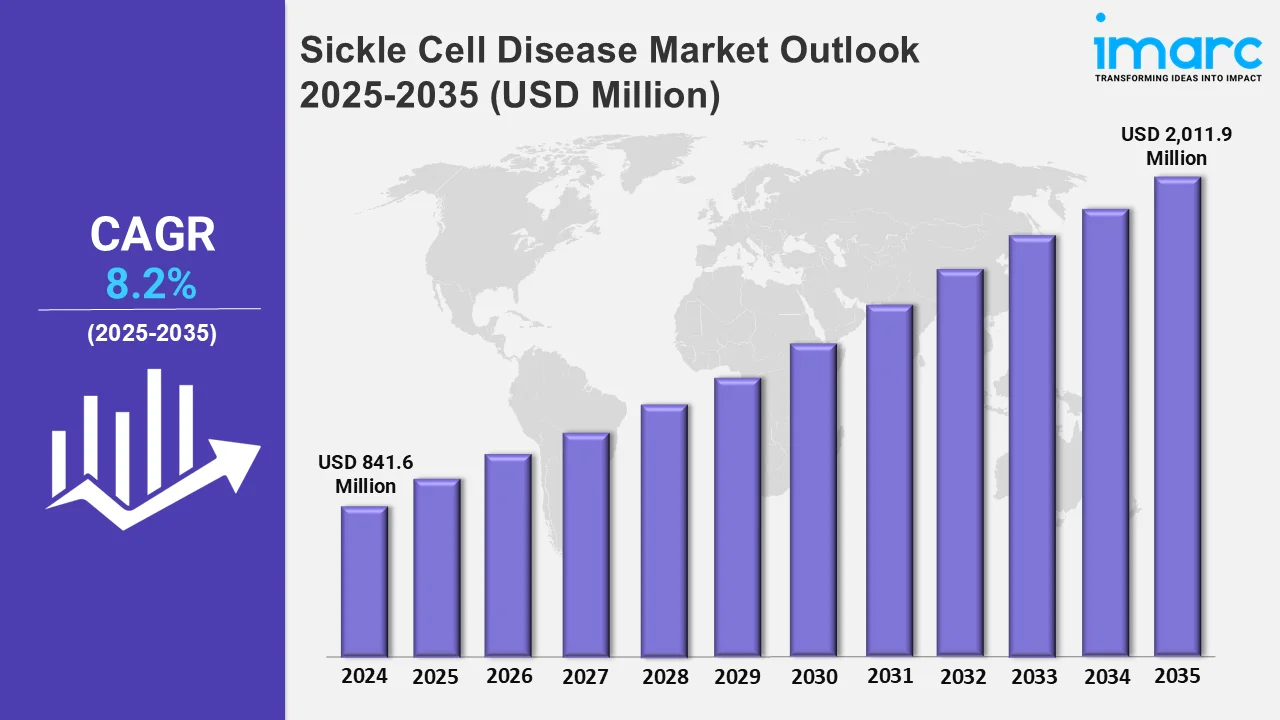
The sickle cell disease market reached a value of USD 841.6 Million in 2024. Looking forward, the market is expected to reach USD 2,011.9 Million by 2035, exhibiting a growth rate (CAGR) of 8.2% during 2025-2035. The market is driven by a robust pipeline of late-stage pharmaceuticals with a high efficacy and safety profile. Gene treatments are the most promising medication possibilities, as they are projected to provide patients with a cure that avoids the pain and risk of a bone marrow transplant.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Sickle Cell Disease Market
The sickle cell disease market is experiencing substantial growth by developments in diagnostic technologies and early detection. Moreover, recent technology advancements have enabled earlier and more precise diagnosis, accelerating the sickle cell disease market's growth. A notable development is the extensive launch of newborn screening initiatives. In order to prevent potentially fatal infections and numerous other problems, early identification enables prompt preventative therapies, such as the administration of penicillin, and patient education. Apart from this, the identification of sickle cell disease is greatly enhanced by developments in molecular diagnostics and genetic testing, which include next-generation sequencing and polymerase chain reaction. These technologies enable not just early diagnosis but also carrier detection, allowing families at risk to make more informed and proactive healthcare decisions. Furthermore, point-of-care testing and mobile diagnostic technologies are becoming more popular, especially in underprivileged areas with limited access to healthcare. By enabling quick screening in remote or low-resource environments, portable diagnostic instruments increase the diagnosis of sickle cell disease. This has a significant impact in places with high incidence but poor healthcare infrastructure, reducing differences in access to care and contributing to market growth.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The market for sickle cell disease is expanding rapidly due to the development of new therapies and pharmaceutical treatments, which offer patients who have had few treatment options in the past new hope. Recent advances in therapeutic techniques are redefining the treatment landscape, with the goal of addressing the disease's underlying cause and improving patient outcomes. Besides this, developing disease-modifying treatments that target the fundamental mechanisms of sickle cell disease rather than just managing its symptoms is one of the most promising areas of innovation. The approval of innovative medications such as crizanlizumab and voxelotor has transformed the market. While Crizanlizumab, a monoclonal antibody, prevents blood vessel cell adhesion and reduces the incidence of excruciating vaso-occlusive crises, Voxelotor increases hemoglobin levels by enhancing red blood cells' ability to retain oxygen. Moreover, gene therapy is another groundbreaking invention with the potential to cure sickle cell disease. CRISPR-Cas9 gene editing and gene addition therapies seek to correct the disease-causing genetic defect or increase the production of healthy hemoglobin. As these gene therapies approach regulatory approval, they are set to transform the sickle cell disease market by offering long-term remedies that go beyond conventional pharmaceutical treatments. Additionally, the rise of small molecule drugs, including JAK inhibitors and other anti-inflammatory agents, is expanding therapeutic options. These drugs target specific pathways involved in inflammation and immune response, offering potential benefits for managing chronic pain and inflammation associated with sickle cell disease.
Regional Analysis:
The major markets for sickle cell disease include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for sickle cell disease while also representing the biggest market for its treatment. This can be attributed to various innovative treatment advances that are changing the course of sickle cell disease.
Moreover, recent approvals of disease-modifying medications, such as crizanlizumab and voxelotor, have provided new alternatives for treating the disease's underlying pathophysiology rather than just its symptoms. The market is also experiencing growth due to the expanding pipeline of gene therapies and curative techniques, such as CRISPR-based treatments, which could provide patients with lifelong solutions.
Besides this, rising awareness and advocacy activities are boosting early diagnosis and access to care, resulting in more effective disease management. Improved healthcare infrastructure and improved insurance coverage for innovative treatments make cutting-edge therapies more accessible, which helps to drive the market growth?.
Key information covered in the report.
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the sickle cell disease market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the sickle cell disease market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current sickle cell disease marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












