Global Pharmaceutical Logistics Market Expected to Reach USD 154.0 Billion by 2033 - IMARC Group
Global Pharmaceutical Logistics Market Statistics, Outlook and Regional Analysis 2025-2033
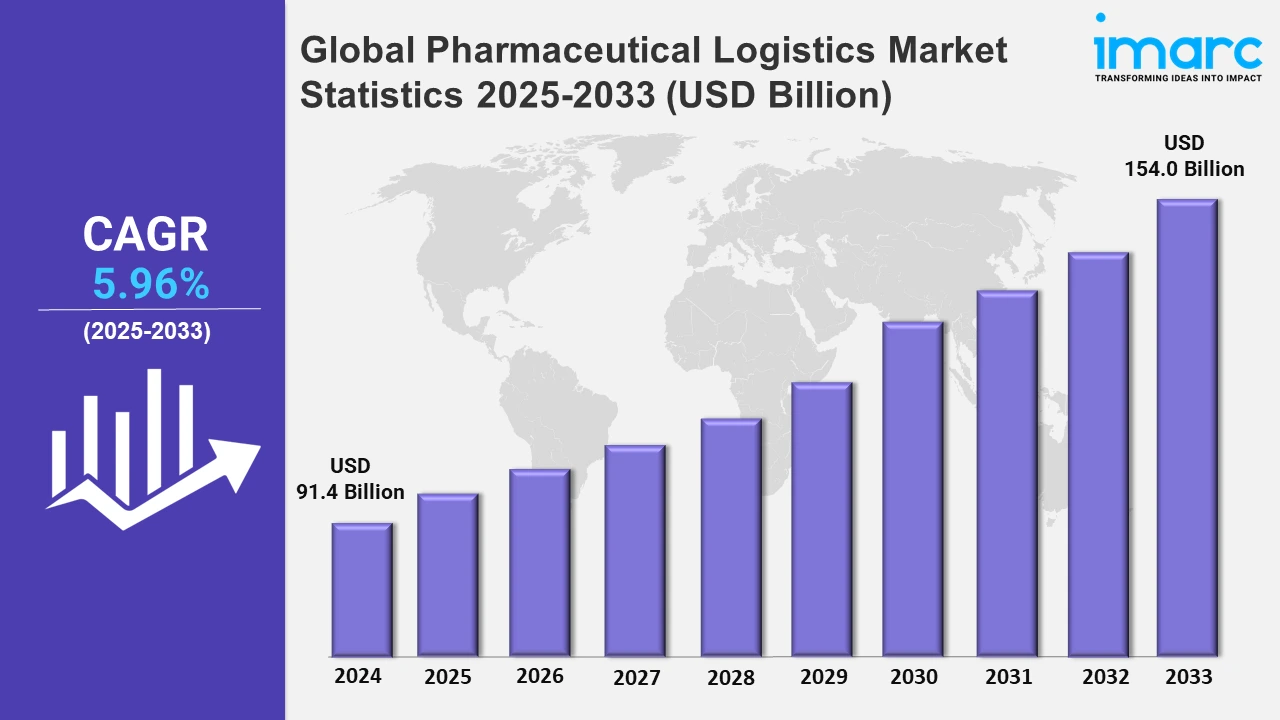
The global pharmaceutical logistics market size was valued at USD 91.4 Billion in 2024, and it is expected to reach USD 154.0 Billion by 2033, exhibiting a growth rate (CAGR) of 5.96% from 2025 to 2033.
To get more information on this market, Request Sample
The growing emphasis on temperature-sensitive logistics solutions is driving the pharmaceutical logistics market, as ensuring the safe transport of medicines and vaccines remains critical. In contrast, companies are innovating to meet regulatory compliance and improve reliability across global supply chains. Notably, temperature monitoring and IoT technology integration are enhancing the efficiency and traceability of sensitive shipments. For instance, in February 2024, Sensitech introduced TempTale GEO X, an advanced IoT temperature monitoring device designed for GxP-compliant, global tracking of temperature-sensitive products across air, ocean, road, and rail. This innovation aligns with the broader trend of ensuring safety and quality in the distribution of pharmaceuticals, which has become a key growth driver. Besides this, the trend toward optimizing logistics and supply chain infrastructure continues to gain momentum, particularly in North America. In March 2024, Noramco established the Noramco Group, merging Halo Pharma and Purisys to reinforce North American pharmaceutical logistics. This strategic move aims to cut logistics expenses, improve regulatory adherence, and boost domestic drug production capabilities. Also, such efforts address long-standing issues related to drug shortages and dependency on inconsistent foreign supplies, positioning North America as a more resilient player in pharmaceutical distribution. Furthermore, these developments reflect the broader industry focus on enhancing logistics operations to support the growing need for secure and efficient delivery of healthcare products.
Moreover, regional investments in specialized facilities are contributing to the evolution of the pharmaceutical logistics sector. For example, UPS launched a pharma-grade cross-docking facility in Hyderabad, India, in October 2024. This facility incorporates cutting-edge temperature control technologies to safeguard pharmaceutical products during distribution. This development supports the handling of biologics and specialty drugs by ensuring regulatory compliance and safety, which are becoming increasingly integral to modern healthcare. The expansion of temperature-controlled infrastructure is essential for addressing the unique challenges associated with transporting high-value and sensitive pharmaceuticals, reinforcing global supply chain resilience.
Global Pharmaceutical Logistics Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Europe (Germany, France, the United Kingdom, Italy, Spain, and others); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, Europe dominates the pharmaceutical logistics market due to its well-established healthcare infrastructure, robust supply chain networks, and continuous technological advancements.
North America Pharmaceutical Logistics Market Trends:
In North America, the market is driven by advanced cold chain solutions essential for biologics and vaccines. On the contrary, the U.S. leads with innovations in temperature-controlled shipping, utilizing IoT sensors for real-time tracking. For instance, Pfizer’s vaccine distribution during the pandemic demonstrated the importance of reliable, temperature-monitored logistics in ensuring timely delivery and maintaining efficacy across vast geographies.
Europe Pharmaceutical Logistics Market Trends:
Europe is the dominating region in the market due to its well-established healthcare infrastructure, robust supply chain networks, and continuous technological advancements. The region benefits from strategic investments by major players that enhance logistics capabilities. In October 2023, UPS Healthcare announced the launch of UPS Pickup Point locations, a new reverse logistics service for health laboratory customers, across the UK, Germany, Italy, France, and Spain. Besides this, Europe’s strict regulatory standards ensure high-quality control and compliance in pharmaceutical transportation. Overall, these factors, combined with ongoing innovations and supportive governmental policies, solidify Europe's leading position in the market.
Asia Pacific Pharmaceutical Logistics Market Trends:
In Asia Pacific, the pharmaceutical logistics market is expanding due to increased pharmaceutical manufacturing and exports, with China and India leading. On the contrary, enhanced infrastructure and tech integration, like blockchain for supply chain transparency, are significant trends. India’s pharmaceutical sector growth, supported by reliable logistics partners, exemplifies the region’s shift toward digital solutions to streamline distribution and ensure compliance with regulatory standards.
Latin America Pharmaceutical Logistics Market Trends:
In Latin America, the pharmaceutical logistics market emphasizes overcoming infrastructural challenges and enhancing last-mile delivery. Brazil’s government initiatives, combined with private sector investment in refrigerated transport, improve accessibility to remote areas. For example, partnerships to upgrade cold storage facilities showcase efforts to strengthen regional capabilities, ensuring vital medicines reach underserved populations while maintaining temperature requirements for potency.
Middle East and Africa Pharmaceutical Logistics Market Trends:
The Middle East and Africa focus on developing robust pharmaceutical logistics to cater to growing healthcare needs. The UAE exemplifies advancements with its strategic position as a logistics hub, integrating AI for efficient route planning. Furthermore, this trend enables improved distribution across countries with challenging terrains, thereby ensuring stable delivery times and compliance with temperature-sensitive shipment protocols for essential pharmaceuticals.
Top Companies Leading in the Pharmaceutical Logistics Industry
Some of the leading pharmaceutical logistics market companies include C.H. Robinson Worldwide Inc., CEVA Logistics, DB Schenker., Deutsche Post AG, DSV A/S, FedEx Corporation, Kuehne + Nagel, Nippon Express Co. Ltd., SF Express Co. Ltd., and United Parcel Service., among many others. In November 2023, Kuehne + Nagel received IATA CEIV Pharma certification for its entire air logistics healthcare network. Moreover, with this, Kuehne + Nagel is positioned as the first logistics company to achieve over 100 CEIV Pharma-certified stations.
Global Pharmaceutical Logistics Market Segmentation Coverage
- On the basis of the type, the market has been bifurcated into non-cold chain and cold chain, wherein non-cold chain represents the most preferred segment. Non-cold chain pharmaceutical logistics refer to the transportation, storage, and distribution of pharmaceutical products that do not require strict temperature control throughout their supply chain.
- Based on the component, the market is categorized into storage (warehouse and refrigerated container), transportation (sea freight logistics, airfreight logistics, and overland logistic), and monitoring (hardware and software), amongst which storage dominates the market. Storage dominates the pharmaceutical logistics market due to the critical need for temperature-controlled facilities ensuring drug potency and safety. Advanced warehousing solutions with climate control and monitoring are essential for regulatory compliance and efficacy.
- On the basis of the application, the market has been divided into bio pharma, chemical pharma, and specialty pharma, wherein chemical pharma represents the most preferred segment. Chemical pharma includes a wide range of medications, such as antibiotics, antivirals, analgesics, and many others.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 91.4 Billion |
| Market Forecast in 2033 | USD 154.0 Billion |
| Market Growth Rate 2025-2033 | 5.96% |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical and Forecast Trends, Industry Catalysts and Challenges, Segment-Wise Historical and Predictive Market Assessment:
|
| Types Covered | Non-Cold Chain, Cold Chain |
| Components Covered |
|
| Applications Covered | Bio Pharma, Chemical Pharma, Specialty Pharma |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | C.H. Robinson Worldwide Inc., CEVA Logistics, DB Schenker., Deutsche Post AG, DSV A/S, FedEx Corporation, Kuehne + Nagel, Nippon Express Co. Ltd., SF Express Co. Ltd., United Parcel Service, etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












