Optimizing Catheter Production: A Comprehensive Cost Analysis

What is Catheter?
Catheters are flexible, tubular medical devices designed to access various body cavities, organs, or blood vessels for diagnostic or therapeutic purposes.
Key Applications Across Industries:
They can be broadly classified into types based on their application, including urinary catheters, cardiac catheters, and intravenous (IV) catheters. Catheters are commonly used in diverse healthcare settings for managing chronic conditions, enabling fluid drainage, delivering medications, or facilitating minimally invasive surgeries.
What the Expert Says: Market Overview & Growth Drivers
According to an IMARC study, the global catheter market was valued at US$ 48.0 Billion in 2024, growing at a CAGR of 4.9% from 2019 to 2024. Looking ahead, the market is expected to grow at a CAGR of approximately 6.4% from 2025 to 2033, reaching a projected value of US$ 84.0 Billion by 2033.
Key drivers shaping the global catheter market include technological advancements, such as the development of hybrid catheters combining diagnostic and therapeutic capabilities; increasing demand for minimally invasive procedures to reduce recovery times and improve patient outcomes; and rising healthcare expenditure in emerging economies, which is expanding access to advanced medical devices. Furthermore, the market benefits from the increasing prevalence of chronic diseases such as cardiovascular disorders and diabetes, which necessitate catheter-based interventions. Together, these factors contribute to the steady growth of the catheter market.
Case Study on Cost Model of Catheter Manufacturing Plant:
Objective
One of our clients has approached us to conduct a feasibility study for establishing a mid to large-scale catheter manufacturing plant in South Africa.
IMARC Approach: Comprehensive Financial Feasibility
We have developed a comprehensive financial model for the plant's setup and operations. The proposed facility is designed with a production capacity of 1,134,000 units of PTA balloon catheter annually.
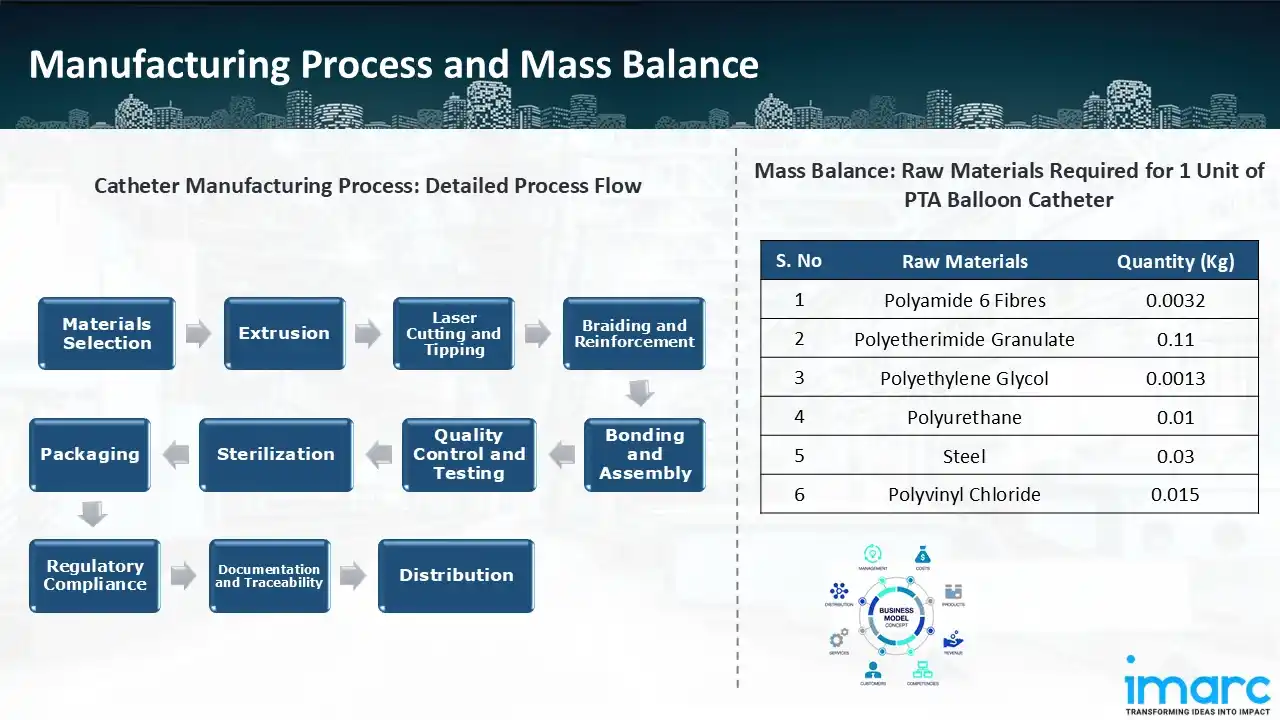
Manufacturing Process: The manufacturing process of catheters begins with materials selection, where biocompatible materials like silicone or polyurethane are chosen for durability and patient safety. For the catheter's tubing to have exact dimensions, the material is extruded. The catheter ends are shaped for best performance using laser cutting and tipping. Additional layers are added during the braiding and reinforcing stage to improve strength and flexibility. After that, the elements are put together using bonding techniques, which guarantee smooth integration of components like balloons or connectors. After rigorous testing and quality control to guarantee adherence to safety regulations, the product is sterilised to prepare it for medical usage. The catheters are packaged under sterile conditions and reviewed for regulatory compliance to meet market requirements. Documentation and traceability ensure each product batch is monitored for accountability. Finally, the catheters are prepared for distribution, ready to be delivered to healthcare providers globally.
Get a Tailored Feasibility Report for Your Project Request Sample
Mass Balance and Raw Material Required: The primary raw materials utilized in the Catheter manufacturing plant include polyamide 6 fibres, polyetherimide granulate, polyethylene glycol, polyurethane, steel, and polyvinyl chloride. To produce 1 unit of PTA balloon catheter, 0.0032 kg of polyamide 6 fibers, 0.1100 kg of polyetherimide granulate, 0.0013 kg of polyethylene glycol, 0.0100 kg of polyurethane, 0.030 kg of steel, and 0.0150 kg of polyvinyl chloride are required.
List of Machinery
The following equipment was required for the proposed plant:
- Solid Silicone Extruder
- Extrusion product mold
- Automatic feeder
- Vulcanization oven
- Oven
- Vertical double slide liquid silicone injection molding machine
- Liquid Silicone Feeder
- Catheter liquid silicone mold
- Catheter liquid silicone mold
- Catheter balloon mold
- Air Cooled Water Chiller
- air compressor
- Dryer
- Air receiver
- Flatbed Vulcanizing Machine
- Kneader
- Catheter balloon mold
- Plastic mold for double lumen catheter body sealing head
- Plastic mold for the sealing head of the three lumen catheter body
- Automatic Cutting Machine
- Main Hole Punching Machine
- Side Hole Punching Machine
- Pad printing
- Rotary Sleeve Machine
- Single Valve Mounting Fixture
- Laser Diameter Measuring Instrument
Techno-Commercial Parameter:
- Capital Investment (CapEx): Capital expenditure (CapEx) in a manufacturing plant includes various investments essential for its setup and long-term operations. It covers machinery and equipment costs, including procurement, installation, and commissioning. Civil works expenses involve land development, factory construction, and infrastructure setup. Utilities such as power, water supply, and HVAC systems are also significant. Additionally, material handling systems, automation, environmental compliance, and safety measures are key components. Other expenditures include IT infrastructure, security systems, and office essentials, ensuring operational efficiency and business growth.
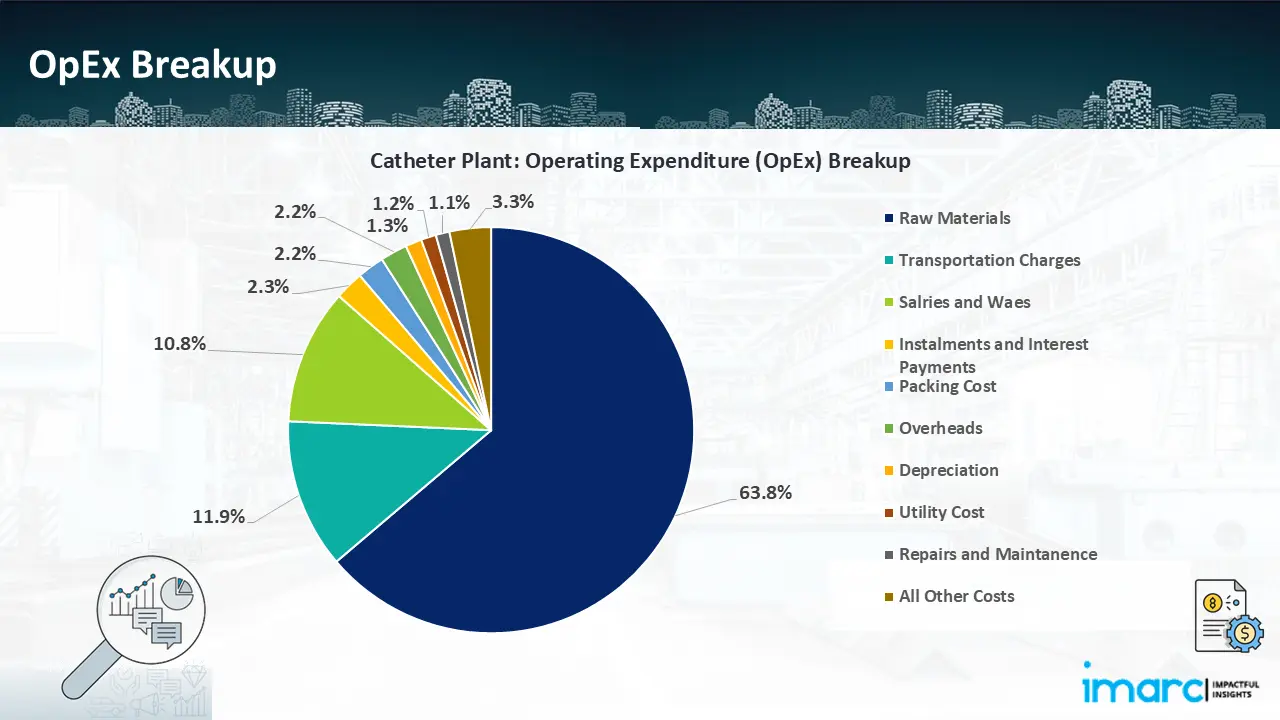
- Operating Expenditure (OpEx): Operating expenditure is the cost incurred to operate a manufacturing plant effectively. OpEx in a manufacturing plant typically includes the cost of raw materials, utilities, depreciation, taxes, packing cost, transportation cost, and repairs and maintenance. The operating expenses are part of the cost structure of a manufacturing plant and have a significant effect on profitability and efficiency. Effective control of these costs is necessary for maintaining competitiveness and growth.
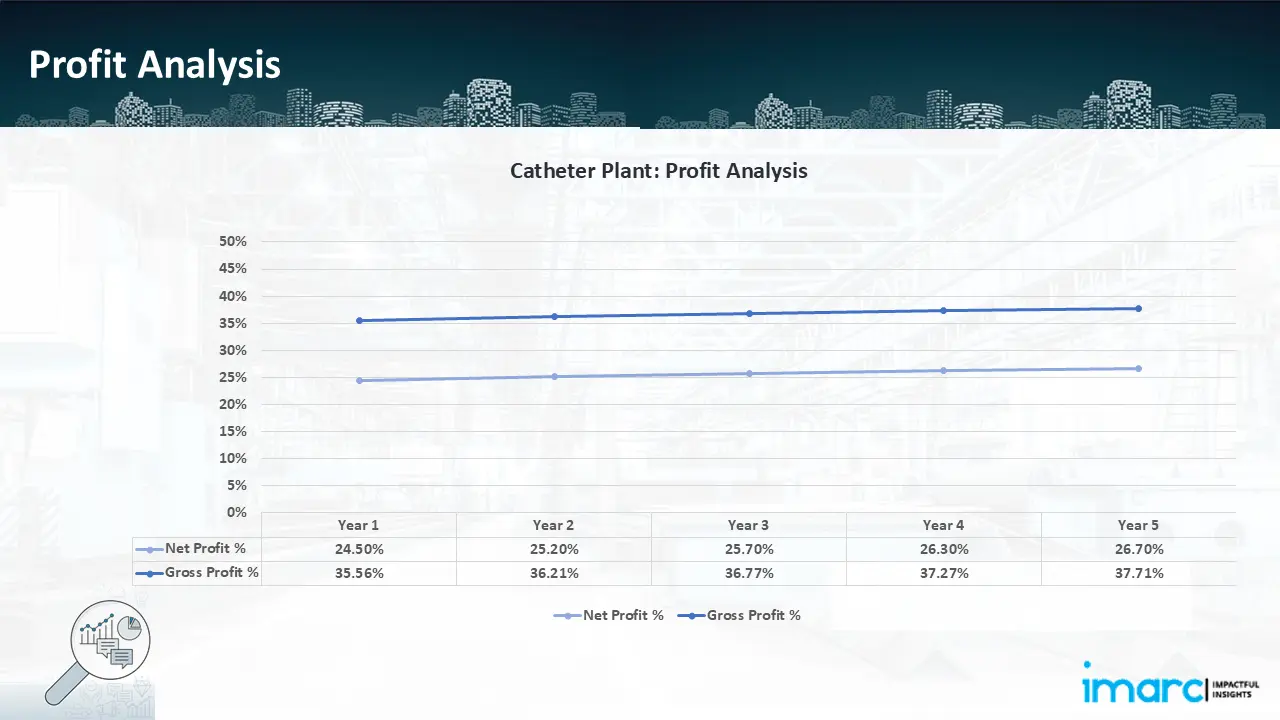
- Profitability Analysis Year on Year Basis: The proposed catheter plant, with a capacity of 1,134,000 units of PTA balloon catheter annually, achieved an impressive revenue of US$ 5.63 million in its first year. We assisted our client in developing a detailed cost model, which projects steady growth, with revenue rising throughout the years. Moreover, gross profit improved from 35.6% to 37.7%, and net profit rise from 24.5% to 26.7%, highlighting strong financial viability and operational efficiency.
Conclusion & IMARC's Impact:
Our financial model for the catheter manufacturing plant was meticulously designed to meet the client’s objectives. It provided a thorough analysis of production costs, including raw materials, manufacturing processes, capital expenditure, and operational expenses. Tailored to the specific requirement of producing 1,134,000 units of PTA balloon catheter annually, the model highlights key cost drivers and forecasts profitability, considering market trends, inflation, and potential fluctuations in raw material prices. This comprehensive financial model offers the client valuable insights for strategic decision-making, demonstrating our commitment to delivering precise, client-focused solutions that ensure the long-term success of large-scale manufacturing projects.
Latest News and Developments:
- In December 2024, Terumo Interventional Systems (TIS), a part of Terumo Corporation, significantly expanded the company's radial-to-peripheral (R2P) portfolio with the announcement of the R2P™ NaviCross® peripheral support catheter's launch and commercial availability in the United States.
- In July 2024, Mexple, a Hyderabad-based business, introduced its ground-breaking UroMen and UroWomen urine collection kits, which come with external urinary catheters made to be comfortable and easy to use for treating incontinence.
- In May 2024, Johnson & Johnson introduced two new devices to improve procedural efficiency in Europe, the Middle East, and Africa: the Cereglide aspiration catheter and the Ethizia haemostatic sealing patch.
- In May 2024, the first international recommendations are released by the World Health Organisation (WHO) to avoid bloodstream infections and other illnesses that may arise from the use of catheters inserted into small blood arteries during medical procedures.
- In May 2024, Johnson & Johnson introduced two new devices to improve procedural efficiency in Europe, the Middle East, and Africa: the Cereglide aspiration catheter and the Ethizia haemostatic sealing patch.
Why Choose IMARC:
IMARC's Financial Model Expertise: Helping Our Clients Explore Industry Economics
IMARC is a global market research company that offers a wide range of services, including market entry and expansion, market entry and opportunity assessment, competitive intelligence and benchmarking, procurement research, pricing and cost research, regulatory approvals and licensing, factory setup, factory auditing, company incorporation, incubation services, recruitment services, and marketing and sales.
Brief List of Our Services: Market Entry and Expansion
- Market Entry and Opportunity Assessment
- Competitive Intelligence and Benchmarking
- Procurement Research
- Pricing and Cost Research
- Sourcing
- Distribution Partner Identification
- Contract Manufacturer Identification
- Regulatory Approvals, and Licensing
- Factory Setup
- Factory Auditing
- Company Incorporation
- Incubation Services
- Recruitment Services
- Marketing and Sales
Under our factory setup services, we assist our clients in exploring the feasibility of their plants by providing comprehensive financial modeling. Additionally, we offer end-to-end consultation for setting up a plant in India or abroad. Our financial modeling includes an analysis of capital expenditure (CapEx) required to establish the manufacturing facility, covering costs such as land acquisition, building infrastructure, purchasing high-tech production equipment, and installation. Furthermore, the layout and design of the factory significantly influence operational efficiency, energy consumption, and labor productivity, all of which impact long-term operational expenditure (OpEx). So, every parameter is covered in the analysis.
At IMARC, we leverage our comprehensive market research expertise to support companies in every aspect of their business journey, from market entry and expansion to operational efficiency and innovation. By integrating our factory setup services with our deep knowledge of industry dynamics, we empower our clients to not only establish manufacturing facilities but also strategically position themselves in highly competitive markets. Our financial modeling and end-to-end consultation services ensure that clients can explore the feasibility of their plant setups while also gaining insights into competitors' strategies, technological advancements, and regulatory landscapes. This holistic approach enables our clients to make informed decisions, optimize their operations, and align with sustainable practices, ultimately driving long-term success and growth.
Our Clients
Contact Us
Have a question or need assistance?
Please complete the form with your inquiry or reach out to us at
Phone Number
+91-120-433-0800+1-201-971-6302
+44-753-714-6104











