Gonorrhea Market Size to Reach USD 1.46 Billion by 2035, Impelled by Growing Burden of Sexually Transmitted Infections
Gonorrhea Market Outlook 2025-2035:
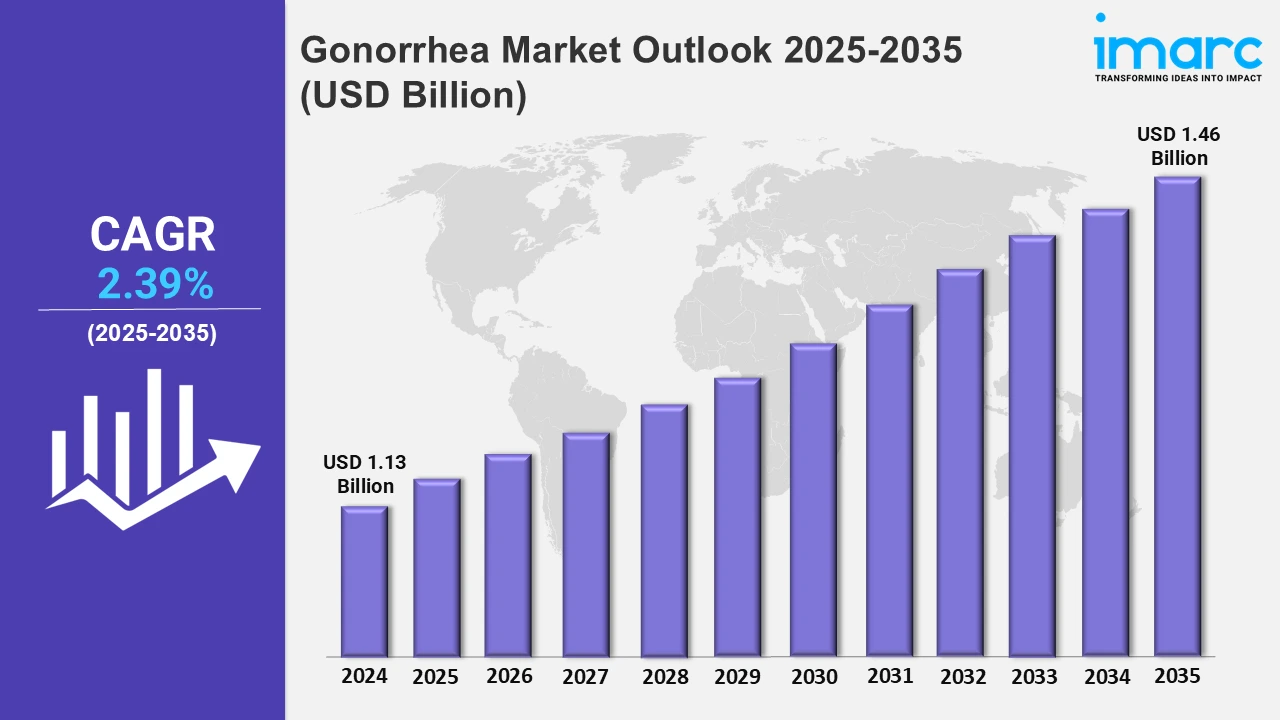
The 7 major gonorrhea market reached a value of USD 1.13 Billion in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 1.46 Billion by 2035, exhibiting a growth rate (CAGR) of 2.39% during 2025-2035. The gonorrhea industry is actively expanding principally because of the rapid rise in occurrence of antibiotic-resistant strains and better awareness of sexually transmitted infections (STIs). Both healthcare and governmental bodies are currently focusing on early diagnosis and treatment, further fueling the market growth. Moreover, numerous pharmaceutical firms are emphasizing on the development of new vaccines as well as antibiotics to mitigate drug resistance issues. Innovations in point-of-care testing are improving early detection rates. Additionally, expanding healthcare infrastructure and better access to treatment in developing regions are further driving market growth.
To get more information on this market, Request Sample
Growing Burden of Sexually Transmitted Infections: Driving the Gonorrhea
The magnifying complications of sexually transmitted infections (STIs) is increasing, with gonorrhea becoming a significant public health concern. Increased urbanization, changing sexual behaviors, and limited consciousness impact its rising prevalence. The widespread use of dating apps and high-risk sexual activities further drive transmission. Alarmingly, the rise of antimicrobial resistance (AMR) in Neisseria gonorrhoeae strains complicates treatment, leading to persistent infections and higher healthcare costs. Inadequate access to screening and stigma surrounding STIs delay early diagnosis and intervention. Additionally, increased travel facilitates cross-border transmission, worsening the spread. Public health initiatives and awareness campaigns remain crucial to reducing infection rates. Expanding diagnostic capabilities, investing in novel antibiotics, and promoting safe sex practices are essential to controlling gonorrhea’s impact. Strengthening surveillance systems to monitor resistance patterns and enhance outbreak response is vital. Collaborative efforts between healthcare providers, policymakers, and researchers can drive innovative treatment strategies. Encouraging routine STI screening and integrating sexual health education into public programs will be key to mitigating gonorrhea’s public health burden.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The development of novel therapies and pharmacological treatments is significantly contributing to the expansion of the gonorrhea treatment market. Pharmaceutical companies and research institutions are actively exploring new drug candidates, including novel antibiotics, combination therapies, and bacteriophage-based treatments. The introduction of next-generation cephalosporins and potential non-antibiotic therapies, such as antimicrobial peptides and immunotherapies, is expected to enhance treatment efficacy. Regulatory approvals of new drugs, along with government and global health initiatives, are accelerating drug development pipelines. The growing adoption of precision medicine and genomic approaches for targeted therapy is further transforming the treatment landscape. Additionally, strategic collaborations between biotech firms and healthcare organizations are fostering innovation. Increased public awareness and improved diagnostic techniques are also driving early detection and treatment adoption. Overall, the emergence of novel pharmacological interventions is playing a crucial role in addressing drug resistance and expanding the gonorrhea treatment market.
Marketed Therapies in Gonorrhea Market
Monodox (Doxycycline Hyclate): Aqua Pharmaceuticals
Monodox (Doxycycline Hyclate), developed by Aqua Pharmaceuticals, is a tetracycline antibiotic approved as part of combination therapy for gonorrhea. It helps inhibit bacterial growth and is often prescribed alongside ceftriaxone to improve treatment efficacy against the infection.
Cipro (Ciprofloxacin): Bayer
Cipro (Ciprofloxacin), developed by Bayer, is a fluoroquinolone antibiotic approved for the treatment of gonorrhea. It functions by prohibiting the functioning of both topoisomerase IV and bacterial DNA gyrase enzymes that are requisite for bacterial DNA replication and repair, resulting in cell death.
Emerging Therapies in Gonorrhea Market
Gepotidacin: GlaxoSmithKline
Gepotidacin, developed by GlaxoSmithKline, is a first-in-class, novel triazaacenaphthylene antibiotic that limits or terminates bacterial DNA replication through a unique mechanism targeting DNA gyrase and topoisomerase IV. It is being investigated for the treatment of uncomplicated gonorrhea, including cases resistant to current antibiotics.
Zoliflodacin: Innoviva Specialty Therapeutics (Formerly Entasis Therapeutics)
Zoliflodacin, developed by Innoviva Specialty Therapeutics (formerly Entasis Therapeutics), is a first-in-class oral antibiotic designed to treat uncomplicated gonorrhea. It works by inhibiting bacterial DNA synthesis through a novel mechanism targeting DNA gyrase, making it effective against drug-resistant Neisseria gonorrhoeae strains. Zoliflodacin offers a promising single-dose oral alternative to current injectable treatments, addressing the growing concern of antibiotic resistance.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Gepotidacin | GlaxoSmithKline | DNA gyrase inhibitors | Oral |
| Zoliflodacin | Innoviva Specialty Therapeutics (Formerly Entasis Therapeutics) | DNA gyrase inhibitors | Oral |
Detailed list of emerging therapies in Gonorrhea is provided in the final report…
Leading Companies in the Gonorrhea Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global Gonorrhea market, several leading companies are at the forefront of developing integrated platforms to enhance the management of Gonorrhea. Some of the major players include Roche, Bayer, GlaxoSmithKline, and others. These companies are driving innovation in the Gonorrhea market through continuous research, diagnostic tools, and expanding their product offerings to meet the growing demand for Gonorrhea.
Key Players in Gonorrhea Market:
The key players in the Gonorrhea market who are in different phases of developing different therapies are Roche, Aqua Pharmaceuticals, Melinta Therapeutics, Innoviva Specialty Therapeutics, Bayer, GlaxoSmithKline, Evofem Biosciences, and others.

Regional Analysis:
The major markets for Gonorrhea include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for Gonorrhea while also representing the biggest market for its treatment. Recent advancements in gonorrhea research focus on tackling antibiotic resistance, a growing global concern. Scientists are developing novel treatment options, including zoliflodacin, a first-in-class oral antibiotic currently in late-stage clinical trials. Additionally, research into bacteriophage therapy and CRISPR-based gene editing shows promise for targeting drug-resistant Neisseria gonorrhoeae. Vaccination efforts have also gained traction, with candidates in early-stage trials exploring immune responses to prevent infection. Improved diagnostic techniques, such as rapid molecular testing, are enhancing early detection and treatment strategies. These innovations aim to curb the spread of resistant strains and improve long-term disease management.
Recent Developments in Gonorrhea Market:
- In September 2024, Innoviva Specialty Therapeutics announced that new findings on its investigational drug zoliflodacin, a first-in-class oral, single-dose antibiotic for treating uncomplicated gonorrhea, will be presented as oral sessions at the 2024 Sexually Transmitted Infections Prevention Conference, taking place from September 16-19, 2024, in Atlanta, GA.
- In April 2024, GSK reported positive findings from the crucial gepotidacin’s EAGLE-1 Phase III trial , a promising first-in-class oral antibiotic with an unprecedented mechanism of action for treating rudimentary urogenital gonorrhea (GC) in adults as well as adolescents. These results are set to be showcased on April 30, 2024, at the ESCMID Global Conference in Barcelona, Spain.
- In February 2024, GSK presented positive headline results from the pivotal EAGLE-1 Phase III trial evaluating gepotidacin, a pioneering first-in-class oral antibiotic with a unique mechanism of action for treating basic urogenital gonorrhea in both adults and adolescents. The trial achieved its core efficiency endpoint, demonstrating that gepotidacin (administered orally in two doses of 3,000 mg) was non-inferior to the combination treatment of intramuscular (IM) ceftriaxone (500 mg) plus oral azithromycin (1,000 mg), a widely used regimen for gonorrhea. This conclusion is based on the main endpoint of microbiological response (failure or success) assessed at the Test-of-Cure (ToC) visit, conducted 3 to 7 days post-treatment.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Gonorrhea market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Gonorrhea market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Gonorrhea marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












