Global Gene Therapy Market Expected to Reach USD 17.9 Billion by 2033 - IMARC Group
Global Gene Therapy Market Statistics, Outlook and Regional Analysis 2025-2033
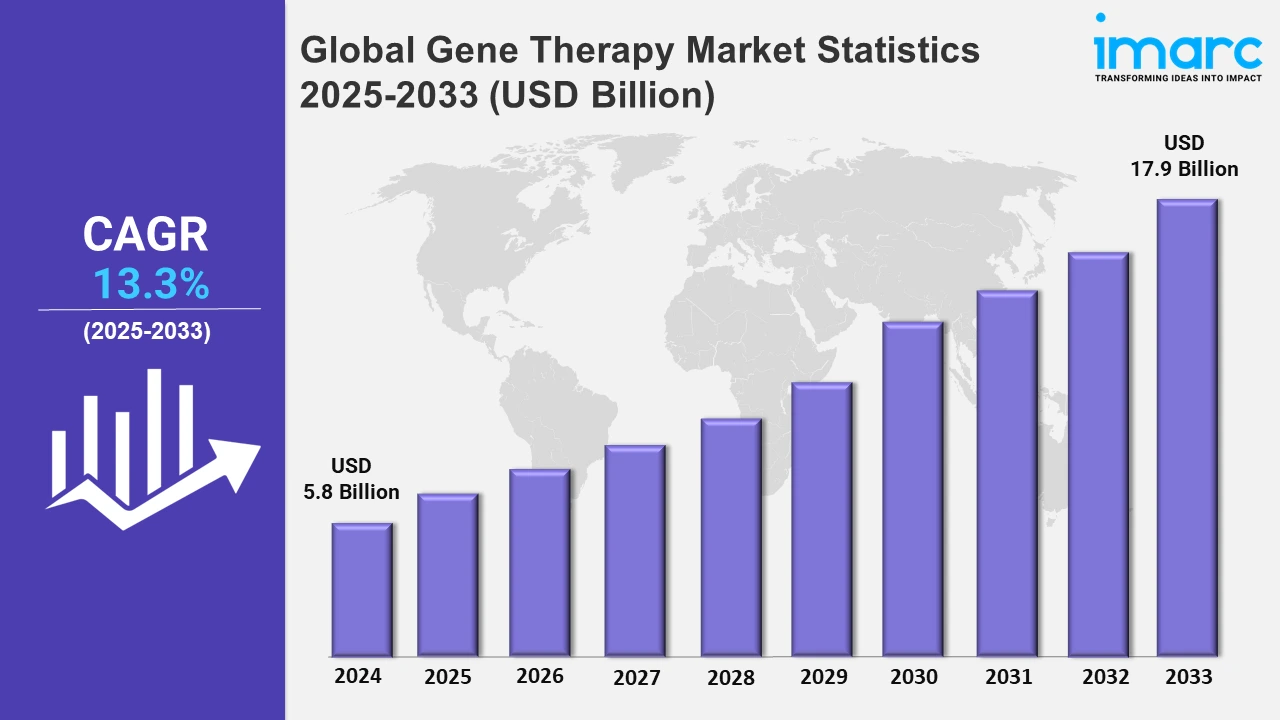
The global gene therapy market size was valued at USD 5.8 Billion in 2024, and it is expected to reach USD 17.9 Billion by 2033, exhibiting a growth rate (CAGR) of 13.3% from 2025 to 2033.
To get more information on this market, Request Sample
Collaborations in gene therapy are helping to advance tailored therapies for rare CNS illnesses. The combination of novel drug delivery technologies with proven gene therapy platforms improves accuracy and efficacy, demonstrating a rising emphasis on merging knowledge to effectively resolve complicated medical challenges. For example, in January 2023, Spark Therapeutics and Neurochase established a strategic collaboration to develop Neurochase’s unique delivery technology for use with selected gene treatments for rare disorders in the CNS. In this agreement, Neurochase would contribute its extensive knowledge in direct drug delivery technology to Spark’s premier AAV platform.
Moreover, FDA approvals for advanced gene treatments demonstrate progress in treating rarely experienced genetic illnesses. The recent approval of a therapy for Duchenne muscular dystrophy indicates expanding attempts to deliver novel therapies for children while improving quality of life through cutting-edge medical developments. For instance, in June 2023, the U.S. FDA granted approval to Sarepta for ELEVIDYS gene therapy to treat DMD in children of age 4-5 years. Furthermore, the gene therapy market is expanding rapidly as precise therapies for relatively uncommon genetic illnesses become more advanced. Companies are focusing on unique delivery modalities to increase efficacy and patient outcomes. Targeted therapies are increasingly chosen over standard treatments because they address genetic factors more precisely. For example, Asia Pacific is developing as a major participant, with Japan approving Luxturna for hereditary retinal diseases, demonstrating the region's regulatory success. Additionally, significant investments in industrial infrastructure, notably in China, are increasing production capacity and accessibility. Collaborations between biotech companies and academic institutes in the region are also encouraging innovation. These advances highlight the global requirement for tailored gene treatments that provide patients with life-changing outcomes. As regulatory frameworks develop and investments increase, the market's potential to transform current medicine and healthcare solutions grows.
Global Gene Therapy Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest gene therapy market share, on account of a robust healthcare infrastructure, a well-established biotechnology industry, and significant investment in gene therapy research and development (R&D) activities.
North America Gene Therapy Market Trends:
North America holds the largest share of the market, owing to expanding gene therapy manufacturing capacity to meet escalating worldwide demand. Companies are developing improved facilities for the scalable manufacture of genetic medicines, as indicated by cutting-edge manufacturing centers in the U.S. Investments in infrastructure and labor development improve the region's capacity to generate high-quality gene medicines, assuring universal access and preserving its leadership in the global gene therapy market.
Europe Gene Therapy Market Trends:
Europe progresses in the industry through regulatory measures, such as the European Medicines Agency's PRIME program, which accelerates medication approvals. Libmeldy, which targets metachromatic leukodystrophy, is one of the successful treatments. Growing alliances among biotech corporations, academia, and healthcare institutions improve access to treatments. The region's emphasis on extending clinical trials and infrastructure development promotes innovation, meeting unmet medical needs and consolidating its position as a hub for genetic discoveries.
Asia-Pacific Gene Therapy Market Trends:
Asia-Pacific sees remarkable growth in the gene therapy market with the expanding R&D activities and supportive regulations in countries like Japan and China. Japan’s approval of Luxturna for retinal disorders demonstrates the region’s commitment to advanced therapies. Increasing investments in gene therapy manufacturing and rising demand for accessible treatments propel Asia Pacific’s significance, addressing genetic disorders and enhancing the global landscape of innovative healthcare solutions.
Latin America Gene Therapy Market Trends:
Latin America advances gene therapy through public-private collaborations and regional innovation. Brazil, a regional pioneer, has integrated CAR-T cell therapy for cancer treatment, showing advances in customized medicine. Efforts to improve infrastructure and transfer technologies boost regional capacities. As awareness and investment increase, Latin America tackles healthcare inequities, establishing itself as an emergent player in the global gene therapy industry.
Middle East and Africa Gene Therapy Market Trends:
The Middle East and Africa region prioritizes gene therapy through early-stage R&D activities and strategic partnerships. Saudi Arabia's Vision 2030 encourages investments in biotechnology to combat hereditary illnesses such as sickle cell anemia. Partnerships with global institutions improve knowledge transfer and infrastructure. While still in its early stages, the region's commitment to advanced medical solutions makes it a prospective place for future growth in the gene therapy industry.
Top Companies Leading in the Gene Therapy Industry
Some of the leading gene therapy market companies include Abeona Therapeutics Inc., Adverum Biotechnologies, Inc., Amgen Inc., Astellas Pharma Inc., BioMarin Pharmaceutical Inc., bluebird bio, Inc., Gilead Sciences, Inc., Mustang Bio, Novartis AG, Orchard Therapeutics plc, Roche Holding AG, Sangamo Therapeutics, and uniQure NV, among many others. For example, in January 2023, Voyager Therapeutics Inc. and Neurocrine Biosciences entered into a strategic collaboration for the commercialization and development of Voyager’s GBA1 program and other next-generation gene therapies for neurological diseases.
Global Gene Therapy Market Segmentation Coverage
- On the basis of the gene type, the market has been bifurcated into antigen, cytokine, tumor suppressor, suicide gene, deficiency, growth factors, receptors, and others. Antigen genes control immune responses, and cytokine genes control inflammation. Tumor suppressor genes restore cellular control, preventing aberrant development, while suicide genes activate processes that destroy cancer cells on a targeted basis. Deficiency genes treat genetic abnormalities, growth factors stimulate tissue repair, and receptor genes influence cellular communication.
- Based on the vector type, the market is categorized into viral vector (adenoviruses, lentiviruses, retroviruses, adeno-associated virus, herpes simplex virus, poxvirus, vaccinia virus, and others) and non-viral techniques (naked and plasmid vectors, gene gun, electroporation, lipofection, and others), amongst which viral vector dominates the market. Viral vectors, such as adeno-associated viruses (AAVs) and lentiviruses, are gaining popularity because they efficiently transport therapeutic genes to target cells.
- On the basis of the delivery method, the market has been divided into in-vivo gene therapy and ex-vivo gene therapy. In-vivo gene therapy is the process of putting genetic material directly into a patient's body in order to successfully cure particular illnesses. Ex-vivo gene therapy modifies cells outside the body before reintroducing them, resulting in precise and individualized therapies for a wide spectrum of hereditary illnesses.
- Based on the application, the market is bifurcated into oncological disorders, rare diseases, cardiovascular diseases, neurological disorders, infectious diseases, and others, wherein oncological disorders dominate the market on account of the ongoing research and development (R&D) activities of gene therapies for oncological disorders.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 5.8 Billion |
| Market Forecast in 2033 | USD 17.9 Billion |
| Market Growth Rate 2025-2033 | 13.3% |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Gene Types Covered | Antigen, Cytokine, Tumor Suppressor, Suicide Gene, Deficiency, Growth Factors, Receptors, Others. |
| Vector Types Covered |
|
| Delivery Methods Covered | In-Vivo Gene Therapy, Ex-Vivo Gene Therapy |
| Applications Covered | Oncological Disorders, Rare Diseases, Cardiovascular Diseases, Neurological Disorders, Infectious Disease, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Abeona Therapeutics Inc., Adverum Biotechnologies, Inc., Amgen Inc., Astellas Pharma Inc., BioMarin Pharmaceutical Inc., bluebird bio, Inc., Gilead Sciences, Inc., Mustang Bio, Novartis AG, Orchard Therapeutics plc, Roche Holding AG, Sangamo Therapeutics, uniQure NV, etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Browse IMARC Related Reports on Gene Therapy Market:
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












