Global Epilepsy Drugs Market Expected to Reach USD 4.6 Billion by 2033 - IMARC Group
Global Epilepsy Drugs Market Statistics, Outlook and Regional Analysis 2025-2033
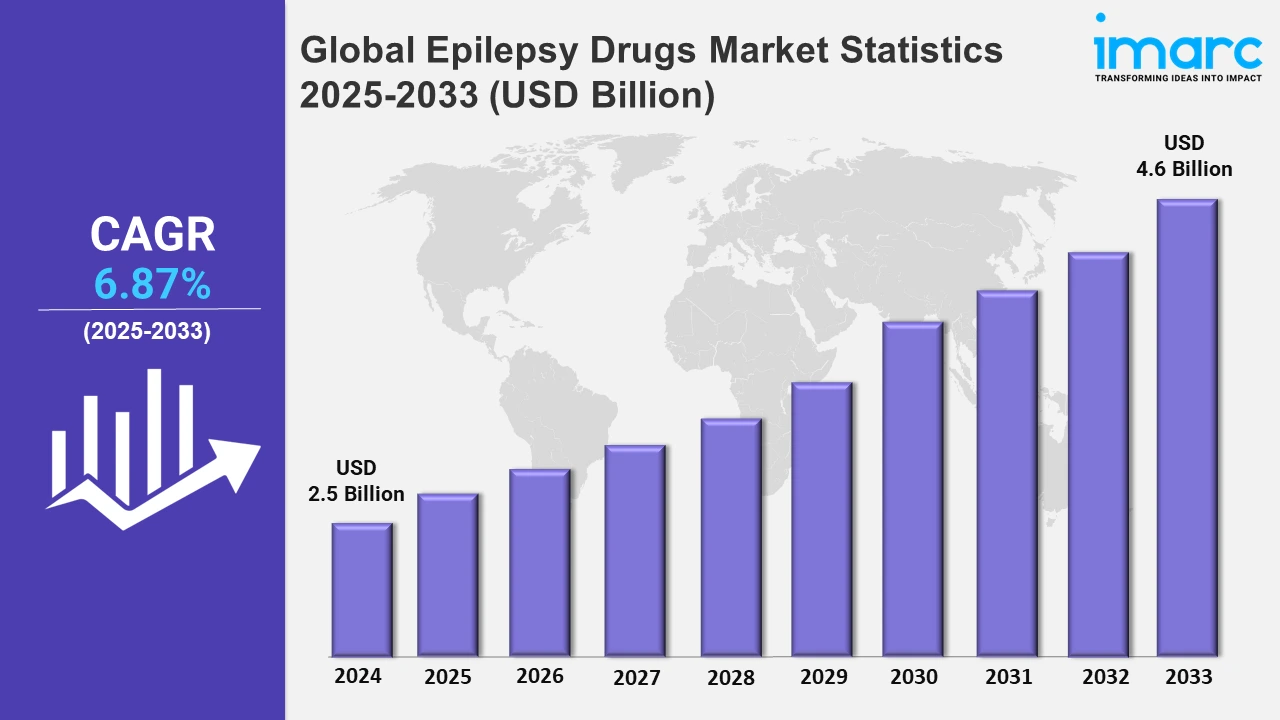
The global epilepsy drugs market size was valued at USD 2.5 Billion in 2024, and it is expected to reach USD 4.6 Billion by 2033, exhibiting a growth rate (CAGR) of 6.87% from 2025 to 2033.
To get more information on this market, Request Sample
The increasing number of epilepsy cases worldwide due to neurological disorders, genetic conditions, and brain injuries represents one of the key factors impelling the market growth. Moreover, public and private sector collaborations focusing on epilepsy awareness, early diagnosis, and access to medication are contributing to the market growth. These initiatives reduce the treatment gap and ensure medications reach underserved regions. Additionally, the development and launch of specialized drugs targeting rare and severe forms of epilepsy are strengthening the market growth. These therapies cater to patients unresponsive to conventional treatments, addressing critical gaps in epilepsy management. By focusing on specific conditions, these medications provide effective solutions for underserved populations, thereby supporting the market growth. For instance, in 2024, For example, in 2024, Akumentis Healthcare introduced Clasepi, a cannabidiol (CBD) medication approved by DCGI for treating epilepsy in India. It is uniquely developed for seizures associated with Lennox-Gastaut Syndrome (LGS), Dravet Syndrome, and Tuberous Sclerosis Complex (TSC) in individuals who are 1 year old and above. Clasepi targets instances when traditional anti-seizure drugs do not work.
Furthermore, the implementation of cutting-edge technologies such as artificial intelligence (AI) and computational modeling is facilitating quicker and more effective drug discovery methods. These technologies enable more precisely targeted treatments and faster launch of novel medications into the market. Besides this, the launch of advanced formulations, such as intravenous options for patients unable to take oral medications, is propelling the market growth. These developments address critical unmet medical needs, offering alternative administration methods for better seizure management. These innovations enhance accessibility for various patient populations, improving treatment adherence and results. In 2024, Eisai Co., Ltd. introduced the intravenous version of Fycompa® (perampanel hydrate) in Japan for epilepsy patients who are unable to take oral medication for a limited time. This groundbreaking AMPA receptor antagonist meets crucial medical requirements, providing an essential option beyond oral treatment for controlling seizures. The drug is included in the Drug Price List of Japan's National Health Insurance (NHI).
Global Epilepsy Drugs Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share, driven by advanced healthcare infrastructure, widespread awareness, robust R&D investments, and the availability of innovative treatment options.
North America Epilepsy Drugs Market Trends:
North America is the largest segment in the market, driven by advanced healthcare systems and a strong focus on neurological disorder management. The area gains from extensive awareness campaigns and access to advanced diagnostic technologies, facilitating prompt and precise treatment. A significant occurrence of epilepsy, along with robust backing for research and development, encourages the emergence of new treatments. Furthermore, the existence of major pharmaceutical firms and efficient regulatory systems guarantees a steady supply of effective drugs, solidifying North America's top status in the market. In 2024, Paladin Labs, a division of Endo International, introduced XCOPRI® (cenobamate tablets) in Canada to serve as an add-on therapy for adults experiencing partial-onset seizures due to epilepsy. The medication targets unfulfilled requirements in epilepsy treatment and has gained positive endorsements from Canadian health officials. Paladin Labs is striving to guarantee broad patient access throughout Canada.
Asia-Pacific Epilepsy Drugs Market Trends:
The Asia-Pacific epilepsy drugs market is witnessing rapid growth with improved accessibility to medical care and an increased focus on the treatment of epilepsy. Governments in the region are implementing policies related to price subsidies for essential drugs, thereby making the treatment more available to more people. Improved health awareness through government and non-governmental services is increasing diagnoses and rates of early intervention in rural areas and other inaccessible regions as well. Additionally, collaboration between healthcare professionals, pharmaceutical companies, and distribution networks is streamlining supply chains, making essential medications more accessible to patients in all geographic locations. Increasing rates of epilepsy due to age and lifestyle-related neurological disorders are major drivers for this demand and encourage innovations and targeted therapy that focus on different kinds of seizures.
Europe Epilepsy Drugs Market Trends:
The European epilepsy drugs market benefits from robust healthcare systems and increased focus on neurological disorder management. Government initiatives and collaborations among healthcare providers drive awareness and access to treatments. The area is experiencing an increase in prescription rates owing to progress in diagnostic technologies and tailored treatment choices. Moreover, continuous research and development efforts by pharmaceutical firms guarantee the accessibility of effective drugs customized for various patient requirements, strengthening Europe’s role in the market.
Latin America Epilepsy Drugs Market Trends:
The market for epilepsy drugs in Latin America is expanding consistently, bolstered by initiatives aimed at enhancing healthcare access and increasing awareness of neurological disorders. Cooperative efforts between governments and healthcare institutions on a regional level enhance diagnosis and treatment programs. Pharmaceutical firms are prioritizing the launch of cost-effective alternatives to meet the requirements of the local community. Rising investments in healthcare facilities and the incorporation of epilepsy management initiatives boost treatment accessibility, aiding market expansion in this area.
Middle East and Africa Epilepsy Drugs Market Trends:
The epilepsy drugs market in the Middle East and Africa displays growth, driven by heightened focus on neurological health and improved treatment access. Joint efforts by international organizations and local authorities are enhancing the accessibility of antiepileptic medications. Initiatives to improve medical education and diagnostic skills are allowing for the earlier and more precise detection of epilepsy instances. Through continued investments in healthcare systems, the area is slowly experiencing improved disease management and increased market prospects.
Top Companies Leading in the Epilepsy Drugs Industry
Some of the leading epilepsy drugs market companies include Abbott Laboratories, Alkem Laboratories Limited, Bausch Health Companies Inc., Eisai Co. Ltd., GSK plc, H. Lundbeck A/S, Jazz Pharmaceuticals plc, Novartis AG, Pfizer Inc., Sunovion Pharmaceuticals Inc. (Sumitomo Dainippon Pharma Co. Ltd.), and UCB S.A., among many others. In 2024, UCB's epilepsy medication Briviact (Brivaracetam) was granted marketing authorization in Japan for the treatment of partial onset seizures in adults with epilepsy. The authorization permits both monotherapy and adjunctive therapy, initiating treatment at a therapeutic dose from day one. This signifies Briviact's fourth approval in the Asia-Pacific region.
Global Epilepsy Drugs Market Segmentation Coverage
- On the basis of the generation type, the market has been categorized into first generation drugs (phenytoin, carbamazepine, oxcarbazepine, valproate, ethosuximide, primidone, and phenobarbital), second generation drugs (levetiracetam, lamotrigine, topiramate, pregabalin, rufinamide, and zonisamide), and third generation drugs (lacosamide, perampanel, eslicarbazepine acetate, and ezogabine/retigabine). Among these, second generation drugs account for the majority of the market share. Second-generation drugs lead the market because of their greater effectiveness and better safety profiles when compared to first-generation alternatives. Drugs such as levetiracetam, lamotrigine, topiramate, and pregabalin are commonly chosen for their effectiveness in controlling seizures with reduced side effects. Their wide-ranging effectiveness renders them appropriate for managing different types of seizures, boosting their use among healthcare professionals. Moreover, these medications are frequently employed as primary treatments, reinforcing their dominance in the market. Their significant market share reflects the growing patient preference and physician trust in their reliability for managing epilepsy.
- Based on the anti-epileptics drugs type, the market is bifurcated into narrow-spectrum AEDs and broad-spectrum AEDs, amongst which broad-spectrum AEDs dominate the market. Broad-spectrum AEDs hold the biggest market share owing to their versatility in treating multiple seizure types and their effectiveness across a wide patient population. Their ability to address both focal and generalized seizures with fewer restrictions on usage contributes to their higher adoption rates. These drugs are preferred for their comprehensive seizure management capabilities, which streamline treatment approaches and enhance patient outcomes, driving their dominance in the market.
- On the basis of the distribution channel, the market has been divided into hospital pharmacy, pharmacy stores, and others, wherein hospital pharmacy represents the leading segment. Hospital pharmacy dominates the market as it is the primary point of distribution for prescribed medications, especially for newly diagnosed patients or those requiring specialized care. Its integration within healthcare facilities ensures immediate access to antiepileptic drugs, facilitating timely treatment. Additionally, hospital pharmacy provides professional guidance from pharmacists, enhancing medication adherence and proper usage, which strengthens their leading position in the market further.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 2.5 Billion |
| Market Forecast in 2033 | USD 4.6 Billion |
| Market Growth Rate 2025-2033 | 6.87% |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Generation Types Covered |
|
| Anti-Epileptics Drugs Types Covered | Narrow- Spectrum AEDs, Broad-Spectrum AEDs |
| Distribution Channels Covered | Hospital Pharmacy, Pharmacy Stores, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Abbott Laboratories, Alkem Laboratories Limited, Bausch Health Companies Inc., Eisai Co. Ltd., GSK plc, H. Lundbeck A/S, Jazz Pharmaceuticals plc, Novartis AG, Pfizer Inc., Sunovion Pharmaceuticals Inc. (Sumitomo Dainippon Pharma Co. Ltd.), UCB S.A., etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












