Cutaneous Lupus Erythematosus Market Size to Reach US$ 373.7 Million by 2034, Impelled by Advancements in Early Detection
Cutaneous Lupus Erythematosus Market Outlook 2024-2034:
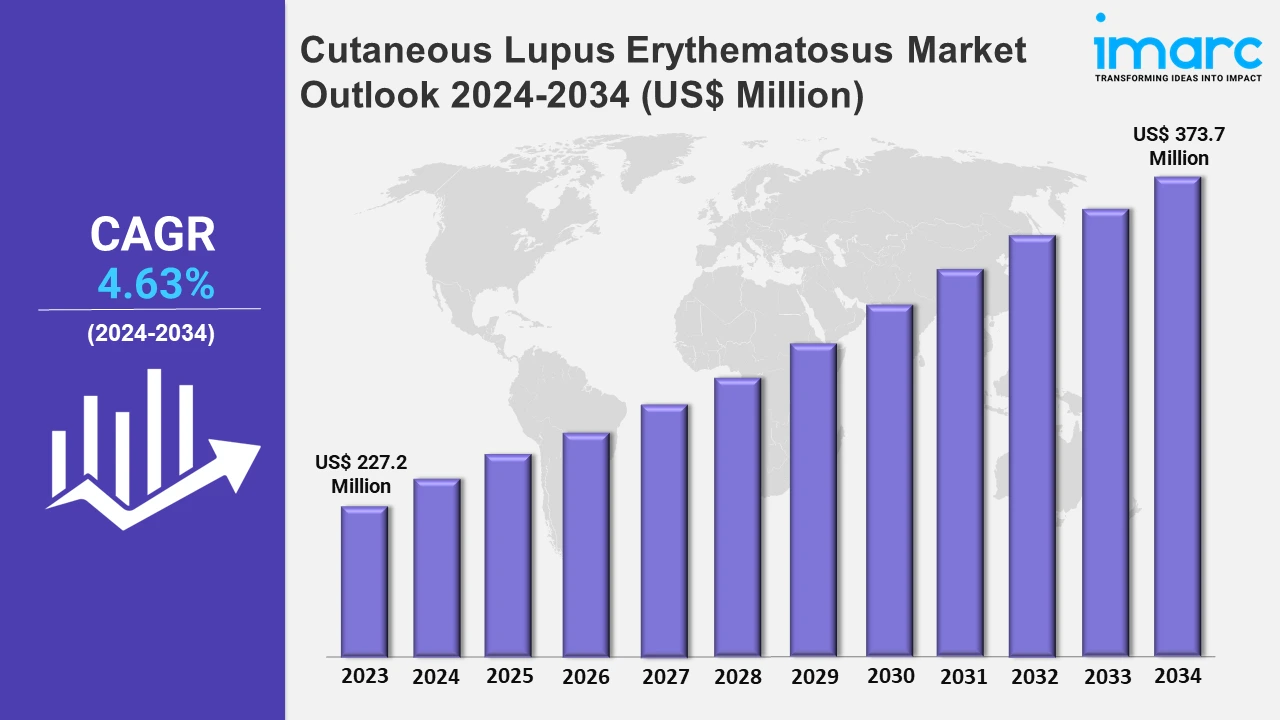
The cutaneous lupus erythematosus market size reached a value of US$ 227.2 Million in 2023. Looking forward, the market is expected to reach US$ 373.7 Million by 2034, exhibiting a growth rate (CAGR) of 4.63% during 2024-2034. The market is driven by the emerging popularity of topical treatments, such as calcineurin inhibitors on account of their targeted approach and fewer systemic side effects in patients.
To get more information on this market, Request Sample
Advances in Early Detection and Diagnostic Technologies: Driving the Cutaneous Lupus Erythematosus Market
Advances in early detection and diagnostic technologies are significantly driving the market for cutaneous lupus erythematosus (CLE). Several innovations in diagnostic tools have enhanced the ability to detect CLE at earlier stages, boosting market growth. One of the key advances in early detection is the use of immunologic biomarkers to identify specific autoantibodies associated with lupus, such as anti-nuclear antibodies (ANAs), anti-dsDNA, and anti-Ro/SS-A antibodies. These biomarkers help clinicians diagnose CLE more accurately and differentiate it from other dermatologic conditions with similar symptoms. Additionally, next-generation sequencing (NGS) allows for the identification of genetic predispositions to CLE, enabling more personalized treatment strategies based on a patient's genetic profile. Furthermore, non-invasive imaging techniques like high-resolution ultrasound and dermoscopy are being utilized to assess skin lesions more effectively. These tools provide detailed images of the skin, helping doctors track disease progression and assess treatment responses without the need for invasive biopsy procedures. The growing emphasis on patient-friendly testing and early intervention has spurred demand for advanced diagnostic technologies in the CLE market. As these technologies continue to evolve, they are expected to facilitate more accurate diagnoses, lead to better management of the disease, and ultimately contribute to the growth of the CLE treatment market by fostering timely and more effective therapeutic strategies.
Development of Novel Therapies and Pharmacological Treatments: Contributing to Market Expansion
The development of novel therapies and pharmacological treatments is a key driver in the expansion of the cutaneous lupus erythematosus (CLE) market, addressing unmet medical needs and improving patient outcomes. Historically, treatment for CLE has relied on general immunosuppressive drugs, such as corticosteroids and antimalarial agents like hydroxychloroquine. While effective for many, these treatments often come with long-term side effects and do not address the specific immune mechanisms driving CLE. However, the advent of targeted therapies is revolutionizing the treatment landscape. Drugs such as belimumab, a monoclonal antibody targeting B-lymphocyte stimulator (BLyS), have shown promise in treating systemic lupus, and ongoing research is exploring its potential application in CLE. Other biologics, including rituximab, which targets B-cells, and anifrolumab, targeting type I interferons, are also being investigated for their ability to modulate the immune response more effectively in CLE patients. In addition, Janus kinase (JAK) inhibitors and phosphodiesterase 4 (PDE4) inhibitors are emerging as promising treatments for inflammatory skin diseases, including CLE. These oral therapies can selectively inhibit immune pathways involved in the inflammatory process, offering more convenient alternatives to injectable biologics. The focus on personalized medicine in CLE treatment is also contributing to market expansion. By tailoring therapies based on a patient’s genetic profile and disease subtype, novel treatments offer improved efficacy with fewer side effects. As these innovations advance, they are expected to transform the management of CLE, driving significant growth in the market by offering patients more targeted, effective, and safer treatment options.
Emerging Therapies in Cutaneous Lupus Erythematosus Market
Edecesertib: Gilead Sciences
Edecesertib is a selective small molecule inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK4), developed by Gilead Sciences. IRAK4 is a serine-threonine kinase that regulates signaling pathways including Toll-like receptors (TLRs) and interleukin-1 receptors. Edecesertib is being studied for its safety and tolerability in participants with CLE in a randomized, blinded, placebo-controlled Phase 1b study.
BIIB059 (litifilimab): Biogen
BIIB059 (litifilimab) is a humanized monoclonal antibody developed by Biogen to treat cutaneous lupus erythematosus. BIIB059 interacts with the BDCA2 receptor that is predominantly expressed on a subset of human immune cells called plasmacytoid dendritic cells (pDCs). pDCs generate cytokines that cause inflammation and contribute to lupus. BIIB059 prevents the formation of several pro-inflammatory chemicals, including IFN-I.
M5049: Merck
M5049 is under development by Merck for the treatment of cutaneous lupus erythematosus. M5049 is a Toll-like receptor 7 (TLR7) and TLR8 antagonist. By inhibiting these receptors, which are involved in the activation of the innate immune response, M5049 helps reduce the overproduction of type I interferons and pro-inflammatory cytokines, thereby alleviating inflammation in cutaneous lupus erythematosus.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Edecesertib | Gilead Sciences | IRAK4 protein inhibitors | Oral |
| BIIB059 (litifilimab) | Biogen | Immunomodulators | Subcutaneous |
| M5049 | Merck | Toll-like receptor 7 antagonists; Toll-like receptor 8 antagonists | Oral |
Detailed list of emerging therapies in Cutaneous Lupus Erythematosus is provided in the final report.
Key Players in Cutaneous Lupus Erythematosus Market:
The key players in the Cutaneous Lupus Erythematosus market who are in different phases of developing different therapies are Gilead Sciences, Biogen, Merck, and Others.

Regional Analysis:
The major markets for cutaneous lupus erythematosus include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for cutaneous lupus erythematosus while also representing the biggest market for its treatment. This can be attributed to the ongoing development of novel treatments, particularly targeted therapies and biologics, which are offering more effective and personalized options.
Moreover, emerging drugs like Toll-like receptor (TLR) antagonists, Janus kinase (JAK) inhibitors, and p38 MAPK inhibitors are showing promise in clinical trials, offering more focused mechanisms of action that can reduce skin inflammation without broader immune suppression.
Besides this, the increased availability of diagnostic tools and patient advocacy are improving early diagnosis and treatment, helping to reduce disease progression and improving quality of life. Additionally, the expansion of patient advocacy and lupus-focused research is helping to drive both demand for treatments and the development of new therapies.
Recent Developments in Cutaneous Lupus Erythematosus Market:
- In October 2022, Biogen Inc. reported that the first patient had been dosed in AMETHYST, a global clinical investigation. The Phase 2/3 study will evaluate the clinical efficacy and safety of litifilimab (also known as BIIB059), a first-in-class, humanized IgG1 monoclonal antibody (mAb) targeting blood dendritic cell antigen 2 (BDCA2), as compared to placebo, in participants with cutaneous lupus erythematosus.
Key information covered in the report.
- Base Year: 2023
- Historical Period: 2018-2023
- Market Forecast: 2024-2034
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the cutaneous lupus erythematosus market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the cutaneous lupus erythematosus market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current cutaneous lupus erythematosus marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












