Chronic Obstructive Pulmonary Disease Market Expected to Exhibit a CAGR of 3.49% during 2025-2035. Impelled by Increasing Awareness & Early Diagnosis
Chronic Obstructive Pulmonary Disease Market Outlook 2025-2035:
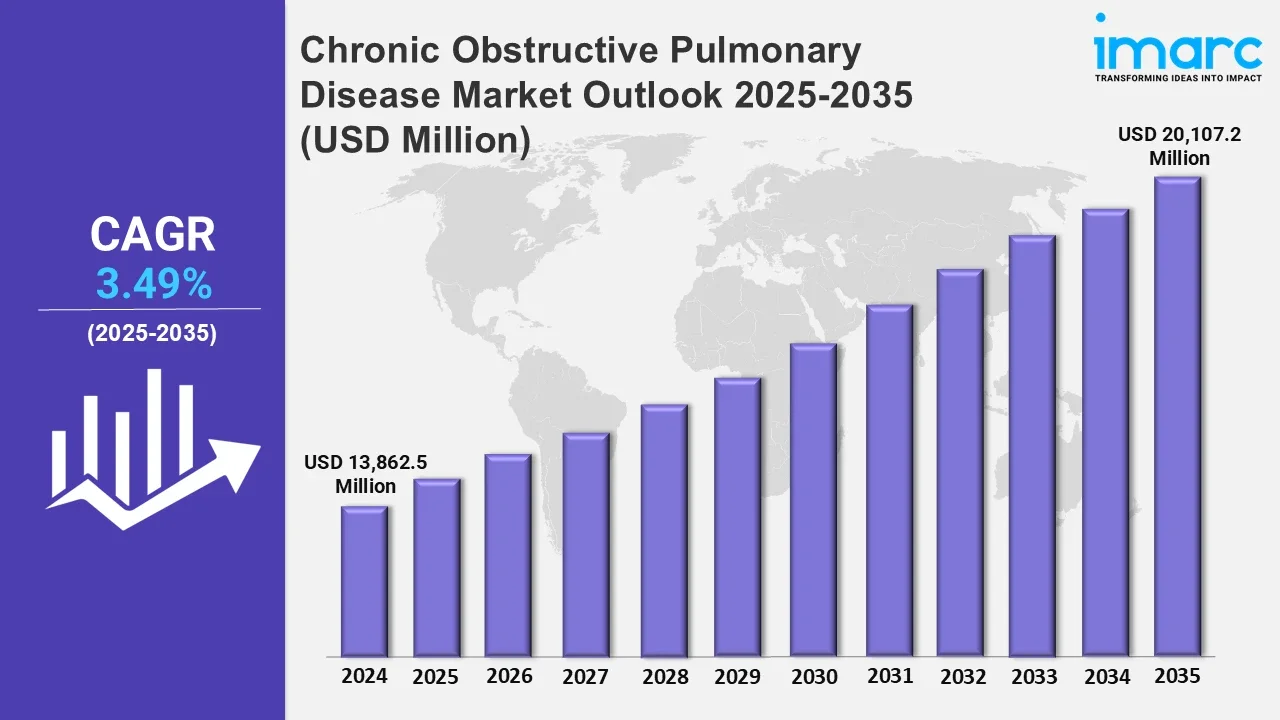
The Chronic Obstructive Pulmonary Disease market reached a value of USD 13,862.5 Million in 2024. Looking forward, IMARC Group expects the 7MM to reach USD 20,107.2 Million by 2035, exhibiting a growth rate (CAGR) of 3.49% during 2025-2035. The COPD treatment market is evolving significantly, led by advances in diagnosis and treatment. A key characteristic of the evolution is a growing emphasis on early and consistent diagnosis, by methods such as pulmonary function tests (PFTs), artificial intelligence (AI) imaging, and biomarker diagnostics. These enable unambiguous disease classification, facilitating patient-tailored treatment regimens and earlier treatment, culminating lastly in improved patient outcomes. Meanwhile, the treatment scenario is being further reshaped by emerging treatment options. Biologic treatments, including IL-5 and IL-13 inhibitors, and long-acting bronchodilators are transforming disease control by minimizing exacerbations and enhancing lung function with fewer adverse effects. Further, the availability of combination treatments, including triple-drug inhalers, bronchodilators plus biologics, and pulmonary rehabilitation programs is enhancing the efficacy of treatments. These advances are improving symptom management, decreasing hospitalizations, and enhancing quality of life, providing new hope for people fighting COPD, a debilitating and irreversible respiratory illness.
To get more information on this market, Request Sample
Rising Incidence and Awareness of Chronic Obstructive Pulmonary Disease (COPD)
The rising prevalence of Chronic Obstructive Pulmonary Disease (COPD) is being fueled by increased awareness as well as the spread of the disease. With more awareness created by public health campaigns and medical interventions, people are approaching early detection, which in turn is causing an increase in the detection rate. Early detection of chronic cough, breathlessness, and wheezing is facilitating the diagnosis of the disease in its early phases, making it easier to manage the disease and also bring about an improved prognosis. Other aspects such as smoking, air pollution, occupational exposures, and genetic susceptibility (e.g., alpha-1 antitrypsin deficiency) are further leading to a rising prevalence of the disease. Heightened recognition by high-risk populations is prompting active screenings and pulmonary function tests (PFTs), resulting in earlier treatment. In line with this, the ongoing government initiatives, research grants, and patient activism are bolstering this trend so that patients receive proper treatment. Ultimately, this higher awareness and earlier diagnosis are leading to improved patient outcomes and improved treatments for COPD.
Expanding Research and Development in COPD Treatment
Pharmaceutical companies are significantly increasing investment in novel drug delivery, biologic treatments, and regenerative medicine for COPD therapy improvement. New-generation bronchodilators, combination inhalers, and anti-inflammatory agents are enhancing disease management and patient results. Biologic therapies against inflammatory pathways like IL-5 and IL-13 inhibitors are in development as emerging therapies for advanced COPD. Gene therapy and stem cell technologies have the potential to introduce disease-altering therapies that can restore lung tissue and stop disease progression. Intelligent inhalers and AI-driven diagnostics are further enhancing medication adherence and personalizing treatments. Cross-collaborations among biotech companies, research institutes, and healthcare organizations are speeding up the development of breakthrough drugs. Consequently, the paradigm for COPD treatment is shifting, providing improved and individualized treatments for patients all over the world.
Marketed Therapies in the Chronic Obstructive Pulmonary Disease Market
Incruse Ellipta (Umeclidinium) – GlaxoSmithKline
Incruse Ellipta is a long-acting muscarinic antagonist (LAMA) from GlaxoSmithKline used for the treatment of COPD for maintenance. It relaxes muscles in the airways, enhances airflow, and alleviates symptoms such as breathlessness.
Yupelri (Revefenacin) - Viatris/Theravance Biopharma
Yupelri is a once-daily, long-acting muscarinic antagonist (LAMA) indicated for maintenance treatment of COPD. It is the first and only once-daily nebulized bronchodilator available for COPD, providing an easy-to-use option for those who have difficulty with inhalers.
Bevespi Aerosphere (Formoterol/glycopyrrolate) – AstraZeneca
Bevespi Aerosphere is a dual bronchodilator containing a long-acting beta-agonist (LABA), Formoterol, and a long-acting muscarinic antagonist (LAMA), Glycopyrrolate for chronic obstructive pulmonary disease (COPD) maintenance therapy. It enhances lung function by calming airway muscles, lessening airflow obstruction and symptoms such as breathlessness.
Striverdi Respimat (Olodaterol) - Boehringer Ingelheim
Striverdi Respimat is a long-acting beta-agonist (LABA) for the treatment of COPD maintenance. It offers 24-hour bronchodilation, assisting in enhancing lung function and lessening symptoms such as breathlessness. Administered through the Respimat inhaler, it provides a fine mist for increased lung deposition and patient convenience.
Emerging Therapies in the Chronic Obstructive Pulmonary Disease Market
Tezepelumab - Amgen/AstraZeneca
Tezepelumab is a monoclonal antibody that is being studied for COPD treatment, acting on thymic stromal lymphopoietin (TSLP) to inhibit airway inflammation. Initially discovered for severe asthma, it has promise in decreasing exacerbations and enhancing lung function in COPD patients with high inflammatory reactions.
| Drug Name | Company Name | MOA | ROA |
|---|---|---|---|
| Tezepelumab | Amgen/AstraZeneca | Thymic stromal lymphopoietin inhibitors | Subcutaneously |
Detailed list of emerging therapies for Chronic Obstructive Pulmonary Disease provided in the final report…
Leading Companies in the Chronic Obstructive Pulmonary Disease Market:
The Chronic Obstructive Pulmonary Disease (COPD) market is witnessing a tremendous shift, fueled by competition and innovation breakthroughs by top pharmaceutical and biotech players. Established players like GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Viatris/Theravance Biopharma, and Amgen are making heavy bets on new bronchodilators, biologic medicines, and combination therapies to enhance the management of COPD. There is a significant transition underway, away from conventional inhaled treatments towards disease-modifying therapies that target airway inflammation, mucus hypersecretion, and lung function loss specifically. This is reflected in the creation of sophisticated biologics such as Tezepelumab, second-generation LAMA/LABA combinations, and regenerative medicine strategies, which seek to improve treatment effectiveness, minimize exacerbations, and enhance quality of life. Facilitated by continuing clinical trials, precision medicine progress, and favorable regulatory environments, the future for COPD therapy is becoming increasingly personalized and targeted, providing hope for more controlled disease and long-term success in millions of patients with this affliction.
Key Players in the Chronic Obstructive Pulmonary Disease Market:
The key players in the Chronic Obstructive Pulmonary Disease market who are in different phases of developing different therapies are GlaxoSmithKline, AstraZeneca, Boehringer Ingelheim, Viatris/Theravance Biopharma, and Amgen, and others.

Regional Analysis:
The market for treating Chronic Obstructive Pulmonary Disease (COPD) is highly concentrated in developed markets including the United States, Germany, the UK, France, Italy, Spain, and Japan, where major growth in respiratory medicine and precision therapies is fueling innovation. The United States is the key to the market with its high incidence of COPD and dominance in diagnostic innovations, new inhaled therapies, and biologic drug development. Although existing treatment strategies emphasize bronchodilators, inhaled corticosteroids (ICS), combination therapy, and oxygen therapy, there is considerable advancement in the comprehension of the molecular and inflammatory processes of COPD, which is translating into targeted and personalized therapies. The COPD treatment market is also being driven by increasing investment in respiratory disease R&D, expedited regulatory approvals for novel therapies, and strategic partnerships among pharmaceutical firms, research organizations, and healthcare providers. These collaborative endeavors are broadening the scope of treatment, enhancing disease control, and increasing quality of life for millions of patients with Chronic Obstructive Pulmonary Disease.
Key information covered in the report
- Base Year: 2024
- Historical Period: 2019-2024
- Market Forecast: 2025-2035
Countries Covered
- United States
- Germany
- France
- United Kingdom
- Italy
- Spain
- Japan
Analysis Covered Across Each Country
- Historical, current, and future epidemiology scenario
- Historical, current, and future performance of the Chronic Obstructive Pulmonary Disease market
- Historical, current, and future performance of various therapeutic categories in the market
- Sales of various drugs across the Chronic Obstructive Pulmonary Disease market
- Reimbursement scenario in the market
- In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current Chronic Obstructive Pulmonary Disease-marketed drugs and late-stage pipeline drugs.
In-Market Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
- Drug Overview
- Mechanism of Action
- Regulatory Status
- Clinical Trial Results
- Drug Uptake and Market Performance
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. Across the six major continents and 100+ countries, we work alongside our business partners as one team with a common ambition to achieve unparallelled results, gain a competitive edge, and transform industries. IMARC Group excels in understanding its clients’ business priorities and delivering tailored solutions that drive meaningful outcomes. Our client base spans over 3,000 organizations in the private, public, and social sectors, ranging from high-growth startups to Fortune 500 companies.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












