Global Cholera Vaccines Market Expected to Reach USD 13.6 Million by 2033 - IMARC Group
Global Cholera Vaccines Market Statistics, Outlook and Regional Analysis 2025-2033
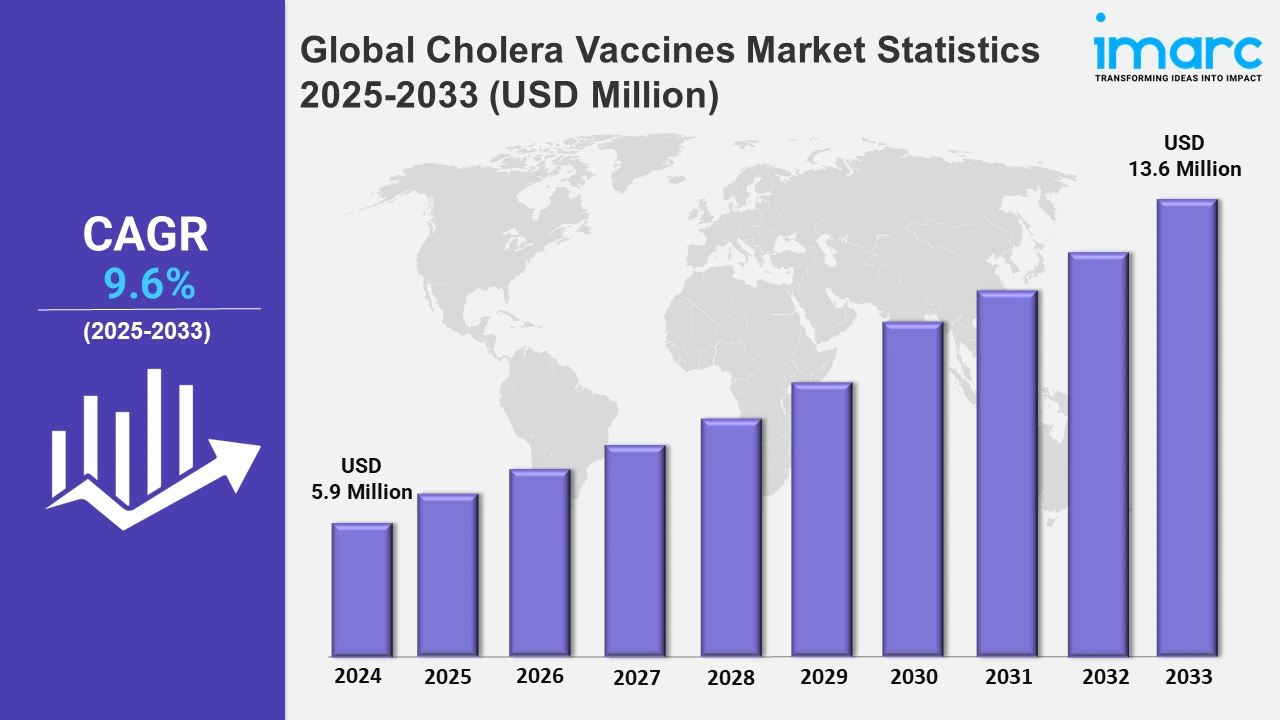
The global cholera vaccines market size was valued at USD 5.9 Million in 2024, and it is expected to reach USD 13.6 Million by 2033, exhibiting a growth rate (CAGR) of 9.6% from 2025 to 2033.
To get more information on this market, Request Sample
The resurgence of cholera outbreaks, particularly in regions with inadequate sanitation and clean water access, has heightened the demand for effective vaccination strategies. For instance, according to the World Health Organization (WHO), in 2022, 44 countries reported cholera cases, up 25% from 35 in 2021. Researchers predicted that 1.3 to 4.0 million cases of cholera occur each year, with 21,000 to 143,000 deaths worldwide. In addition, the number of recorded cholera cases climbed by 13% and deaths by 71% in 2023 compared to 2022. As communities learn how deadly cholera episodes can be, the shift for proper prevention, including vaccines, is accelerating. The growing use of vaccines in cholera prevention and the promotion of mnemonic vaccination is thus expanding the cholera vaccines industry.
Moreover, the continuous improvements in vaccine technology are also key to the expansion of the market. Along with this, newer oral cholera vaccines (OCVs) are offering more efficacious and cost-effective immunization tools. For instance, in August 2024, Bharat Biotech International Ltd launched Hillchol, a unique single-strain oral cholera vaccine, following a successful phase-III clinical trial that validated the jab's safety. BBIL has also constructed large-scale manufacturing facilities in Hyderabad and Bhubaneswar capable of producing up to 200 million doses of Hillchol. Moreover, improvements in the new antiviral drugs are aiding the second-generation vaccines to be even more potent, achieve longer-term protection, and be more convenient to deliver. Their single-dose regimens and capability to maintain in difficult storage conditions for long periods are improving their attractiveness. Besides this, supportive government policies and rising funding from international organizations are one of the major factors driving the market. Along with this, the national authorities across cholera-endemic areas are applying health policies and vaccination plans to control the spread of cholera. For instance, in October 2024, ICMR-NIRBI (National Institute for Research in Bacterial Diseases), formerly NICED, and the State Health Department of Bengal launched a pilot project in Bishnupur block II of South 24 Parganas, a cholera-prone area, utilizing Euvichol-Plus, a vaccine manufactured in Korea. The pilot effort aimed to vaccinate approximately 50,000 people over the next ten days, after which they will receive a second dosage. All vaccinated individuals will be monitored for two years to measure the vaccine's immunogenicity and lifespan.
Global Cholera Vaccines Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa.
North America Cholera Vaccines Market Trends:
The approval and launch of Vaxchora, the first and only oral cholera vaccine in the United States, have significantly boosted the market growth. This vaccine offers single-dose immunization with reduced adverse effects, making it a preferred choice for travelers to cholera-endemic regions.
Europe Cholera Vaccines Market Trends:
The rising number of cholera outbreaks has heightened awareness and demand for preventive measures, including vaccination. For instance, in 2022, 29 cases of cholera were reported by nine EU and EEA countries. This trend underscores the need for effective vaccines to control and prevent the spread of cholera.
Asia Pacific Cholera Vaccines Market Trends:
Innovations in vaccine technology have resulted in more effective and accessible cholera vaccines. Notably, Bharat Biotech's development of the oral cholera vaccine Hillchol, which received regulatory approval in India in August 2024, exemplifies such advancements. The company plans to produce up to 200 million doses annually to address global shortages.
Latin America Cholera Vaccines Market Trends:
Governments in Latin America have been proactive in implementing immunization programs to combat cholera outbreaks. For instance, countries like Brazil and Mexico have developed self-sufficient vaccine production capabilities, meeting 54.2% and 25% of their national vaccine demands, respectively.
Middle East and Africa Cholera Vaccines Market Trends:
The region has witnessed a surge in cholera outbreaks, exacerbated by extreme weather events such as floods and droughts. For instance, between late 2021 and mid-2024, over 6,000 deaths and nearly 350,000 cases were reported in southern and eastern Africa.
Top Companies Leading in the Cholera Vaccines Industry
Some of the leading cholera vaccines market companies include Astellas Pharma Inc., Celldex Therapeutics Inc. (Avant Immunotherapeutics Inc.), Emergent BioSolutions Inc., Eubiologics Co. Ltd., Johnson & Johnson, Merck & Co. Inc, Pfizer Inc., PharmaChoice Canada Inc, Sanofi S.A., Takeda Pharmaceutical Company Limited, and Valneva SE, among many others. For instance, in May 2024, Johnson & Johnson announced to acquire Yellow Jersey Therapeutics, a wholly owned subsidiary of Numab Therapeutics AG. Also, in May 2024, Astellas Pharma Inc. and YASKAWA Electric Corporation signed a memorandum of understanding to commence discussions about forming a new partnership structure to enable the successful implementation of cell and gene therapy automation technology employing robotics.
Global Cholera Vaccines Market Segmentation Coverage
- On the basis of the vaccine type, the market has been bifurcated into whole cell V. cholerae O1 with recombinant B-subunit and killed oral O1 and O139, wherein whole cell V. cholerae O1 with recombinant B-subunit represented the largest segment. The efficacy, oral vaccine preparations, and dual systemic and mucosal immunization efficacy of this formulation are responsible for dominance.
- Based on the product, the market is categorized into dukoral, shanchol, vaxchora, euvichol and euvichol-plus, and others, amongst which dukoral represented the largest segment. Dukoral (developed by Valneva SE) provides dual protection against both cholera and traveler's diarrhea due to enterotoxigenic Escherichia coli (ETEC), which is especially important for those traveling to endemic areas, further driving the segment’s growth.
- On the basis of the end user, the market has been divided into hospitals and clinics, research and academic laboratories, and others. Among these, hospitals and clinics represented the largest segment as they are responsible for making vaccines widely available and getting individuals vaccinated by providing routine immunization services and preparing to respond quickly to an epidemic.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 5.9 Million |
| Market Forecast in 2033 | USD 13.6 Million |
| Market Growth Rate 2025-2033 | 9.6% |
| Units | Million USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Vaccine Types Covered | Whole Cell V. Cholerae O1 with Recombinant B-Subunit, Killed Oral O1 and O139 |
| Products Covered | Dukoral, Shanchol, Vaxchora, Euvichol and Euvichol-Plus, Others |
| End Users Covered | Hospitals and Clinics, Research and Academic Laboratories, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Astellas Pharma Inc., Celldex Therapeutics Inc. (Avant Immunotherapeutics Inc.), Emergent BioSolutions Inc., Eubiologics Co. Ltd., Johnson & Johnson, Merck & Co. Inc, Pfizer Inc., PharmaChoice Canada Inc, Sanofi S.A., Takeda Pharmaceutical Company Limited, Valneva SE, etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.

 Inquire Before Buying
Inquire Before Buying
 Speak to an Analyst
Speak to an Analyst
 Request Brochure
Request Brochure




.webp)




.webp)












