Global Albumin Market Expected to Reach USD 10.5 Billion by 2033 - IMARC Group
Global Albumin Market Statistics, Outlook and Regional Analysis 2025-2033
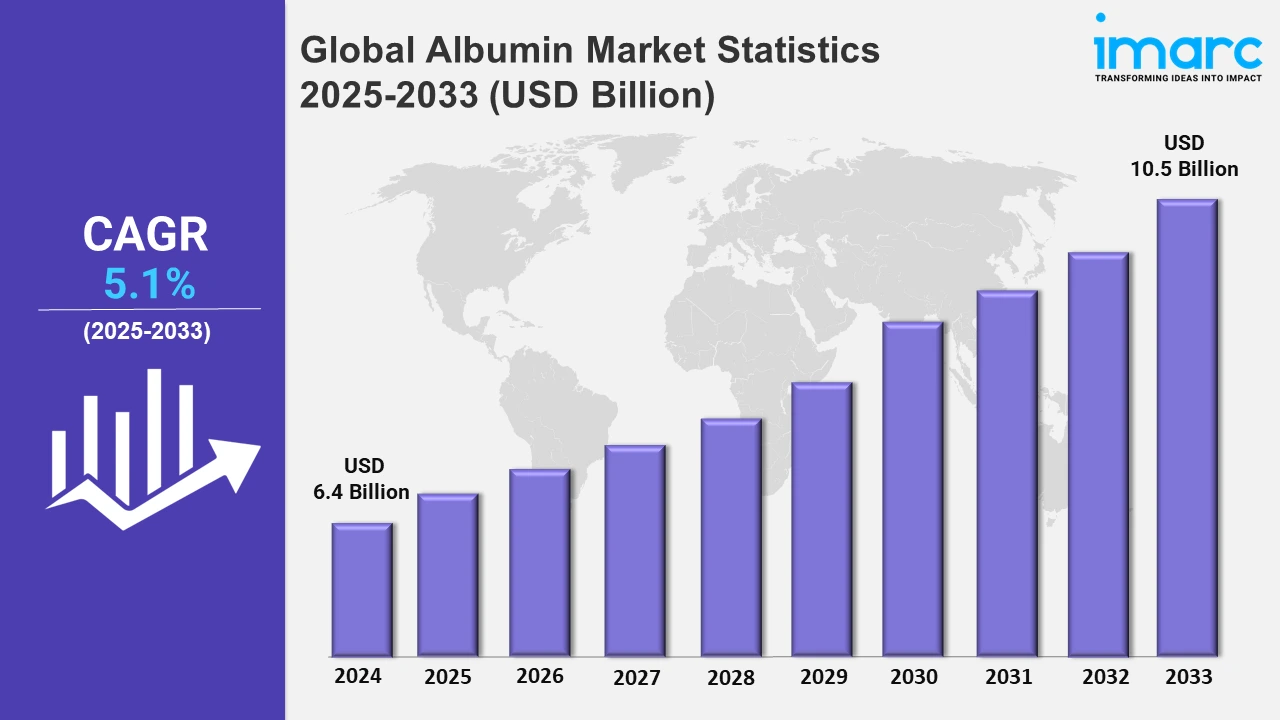
The global albumin market size was valued at USD 6.4 Billion in 2024, and it is expected to reach USD 10.5 Billion by 2033, exhibiting a growth rate (CAGR) of 5.1% from 2025 to 2033.
To get more information on this market, Request Sample
The market is driven mainly by the increasing incidence of chronic diseases, such as cardiovascular conditions, liver diseases, and kidney issues. Besides, conditions such as hypoalbuminemia, in which patients have low levels of albumin in the blood, are mainly treated using albumin. Albumin is very important in therapeutic purposes such as plasma volume expanders, blood substitutes, and drug delivery systems. It is also used in the treatment of burns, surgical procedures, and preparation of vaccines which boosts its demand and clinical usage. The health care industry is growing worldwide, especially in developing countries, where governments are investing more in better health care infrastructures and accessibility of treatments. So, the growth raises a demand for blood plasma-derived proteins such as albumin. In addition, this would mean the diagnostic tests involved for which Albumin is necessary also help boost market growth-its use in liver function tests and evaluation of nutritional status among early-stage diagnostics in personalization. For example, last June 2024, Dyadic International, Inc. issued an announcement stating that the firm has agreed to an open development and commercial partnership with Proliant Health and Biologicals (PHB) leader in purified proteins across diagnostics, nutrition, and cell cultures. The agreement terms also provide that Dyadic will receive an upfront milestone payment of $1.5MM and have agreed to a share of profits received by PHB from the sale of animal-free recombinant albumin products produced using Dyadic's filamentous fungal microbial platforms. Growth in the market is fueled by research aimed at further improving the production of albumin, optimizing its therapeutic effects, and new applications, including drug delivery systems and gene therapy.
Advances in biotechnology, such as recombinant albumin, also underpin this market growth. For instance, first Drug Master File of recombinant Human Albumin 20% was filed with the USFDA by Shilpa Biologicals in June 2024. This new recombinant has been formulated and is a patented process for novel recombinant Human Albumin 20% which is fully environmentally friendly. This product, according to the literature, demonstrates consistent quality, scales up for commercial use, and costs competently. Recombinant Human Albumin 20% attempts to fill an emerging and increasingly growing demand for human serum albumin that can cover today's and future demand. It is the rapidly aging elderly population worldwide that increases vulnerability to chronic diseases requiring the therapy of albumin.
Global Albumin Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia-Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share on account of the high disease prevalence, advanced healthcare infrastructure, increasing therapeutic applications, and aging populations.
North America Albumin Market Trends:
In North America, strong epidemiological factors, including the high incidence of chronic diseases, healthcare infrastructure, and growing demand for therapeutically useful applications, including plasma volume expansion and drug delivery, propel the market growth. Progress in biotechnology, especially recombinant albumin, and the aging population contribute to the growth in this market. For instance, in October 2024, Sandoz, the global leader in generic and biosimilar medicines, announced the launch of a generic paclitaxel formulation in the US, the first generic to its reference medicine. The US Food and Drug Administration (FDA) is expected to approve it as it is considered a breakthrough in the treatment of individuals with metastatic breast cancer. It is expected to be approved by.
Asia-Pacific Albumin Market Trends:
In Asia Pacific, the market is driven by increasing healthcare needs, rising prevalence of chronic diseases, expanding healthcare infrastructure, and growing medical tourism. Additionally, the region’s aging population, advancements in biotechnology, and rising demand for therapeutic treatments such as plasma-derived products and drug delivery systems further boost market growth.
Europe Albumin Market Trends:
In Europe, the market is driven by the rising prevalence of chronic diseases, such as liver and kidney disorders, increased healthcare spending, and aging populations. Additionally, advancements in biotechnology, growing demand for plasma-derived therapies, and regulatory support for biosimilars and recombinant albumin products contribute to market expansion.
Latin America Albumin Market Trends:
The market in Latin America derives from improving health access, increasing chronic disease prevalence, expanding medical infrastructure, and increasing demand for plasma-derived therapies. Apart from this, it is also driven because of the increased awareness regarding the role of albumin in its treatment of conditions such as burns and liver disease.
Middle East and Africa Albumin Market Trends:
In the Middle East and Africa, the albumin market is driven by expanding healthcare infrastructure, rising incidences of chronic diseases, and increasing demand for plasma-derived therapies. Growing awareness of albumin's role in treating conditions like liver disease, burns, and cancer, along with improving access to healthcare, fuels market growth.
Top Companies Leading in the Albumin Industry
Some of the leading albumin market companies include Albumedix Ltd, Biotest AG, Celgene Corporation (Bristol-Myers Squibb Company), CSL Limited, Grifols SA, HiMedia Laboratories, Medxbio Pte Ltd, Merck KGaA, Octapharma AG, Takeda Pharmaceutical Company Limited, Thermo Fisher Scientific Inc., and Ventria Bioscience Inc., among many others.
In May 2024, ARTES, a company specializing in the development and transfer of recombinant cell lines and protein production processes from microbial expression systems, and Basic Pharma, a pharmaceutical company focusing on pharmaceutical products, made significant progress in their strategic collaboration to produce recombinant Human Serum Albumin derived from Hansenula yeast.
Global Albumin Market Segmentation Coverage
- On the basis of the product, the market has been categorized into human serum albumin, bovine serum albumin, and recombinant albumin, wherein human serum albumin represents the leading segment. Human serum albumin accounts for the highest share of the market on account of the wide applications in pharmaceutical treatment, such as the plasma volume expansion, the treatment of burns, and the drug delivery. Its applications are further supported by binding and transporting various substances due to its critical role in conditions like hypoalbuminemia.
- Based on the application, the market is classified into therapeutics, drug formulation and vaccine, component of media, and others, amongst which therapeutics dominate the market. Therapeutics accounts for the majority market share since albumin is critically used for the management of several diseases such as burns, hypoalbuminemia, and liver diseases along with cancer; expansion of plasma volume and use in drug delivery systems besides in the stabilization of biologics.
- Based on the end user, the market has been segmented into hospitals and clinics, pharmaceutical and biotechnology companies, and research institutes. Among these, hospitals and clinics account for the majority of the market share. With the increased demand for albumin in the treatment of serious conditions such as liver diseases, burns, and hypoalbuminemia, hospital and clinic sales dominate the significant market share. These therapeutic settings utilize albumin to achieve therapeutic purposes, increase plasma volume, and deliver drugs, thus increasing consumption and market growth.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 6.4 Billion |
| Market Forecast in 2033 | USD 10.5 Billion |
| Market Growth Rate 2025-2033 | 5.1% |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Products Covered | Human Serum Albumin, Bovine Serum Albumin, Recombinant Albumin |
| Applications Covered | Therapeutics, Drug Formulation and Vaccine, Component of Media, Others |
| End Users Covered | Hospitals and Clinics, Pharmaceutical and Biotechnology Companies, Research Institutes |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Albumedix Ltd, Biotest AG, Celgene Corporation (Bristol-Myers Squibb Company), CSL Limited, Grifols SA, HiMedia Laboratories, Medxbio Pte Ltd, Merck KGaA, Octapharma AG, Takeda Pharmaceutical Company Limited, Thermo Fisher Scientific Inc., Ventria Bioscience Inc., etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.





.webp)




.webp)












