Global ADME Toxicology Testing Market Expected to Reach USD 30.2 Billion by 2033 - IMARC Group
Global ADME Toxicology Testing Market Statistics, Outlook and Regional Analysis 2025-2033
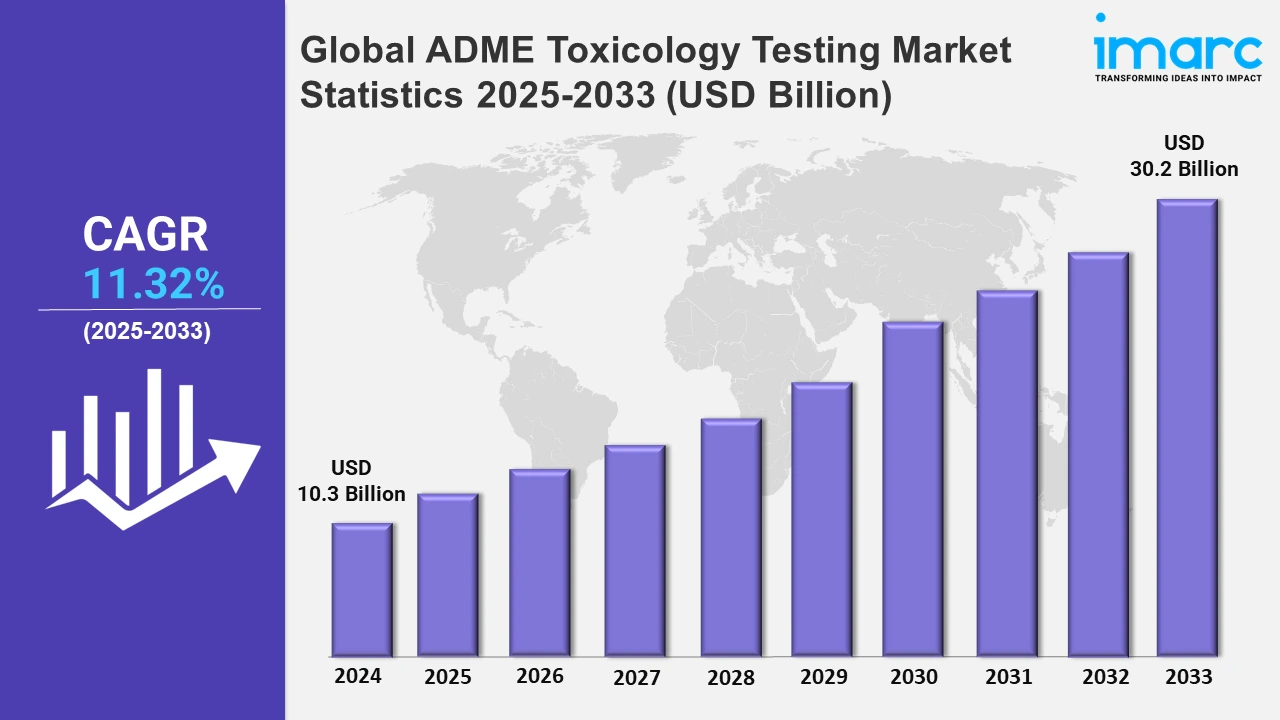
The global ADME toxicology testing market size was valued at USD 10.3 Billion in 2024, and it is expected to reach USD 30.2 Billion by 2033, exhibiting a growth rate (CAGR) of 11.32% from 2025 to 2033.
To get more information on the this market, Request Sample
The global increase in chronic conditions such as cancer, diabetes, and cardiovascular diseases necessitates the development of new therapeutics. For instance, according to the World Health Organization (WHO), in 2022, there were nearly 20 million new cancer cases and 9.7 million fatalities. Cancer affects around one in every five persons in their lifetime, with one in every nine men and one in every twelve women dying from it. Similarly, according to an article published by the American Cancer Society, in 2024, the United States is expected to see nearly 2,001,140 new cancer cases and approximately 611,720 cancer deaths. ADME toxicology testing plays a crucial role in the process of new drug development by ensuring the safety and effectiveness of these pharmaceuticals. As these diseases continue to pose significant health challenges globally, there is a growing need for extensive drug testing to mitigate potential side effects and ensure patient safety. The ADME toxicology tests provide valuable insights into how drugs are absorbed, distributed, metabolized, and excreted by the body, which is vital in the development of effective and safe medications for chronic diseases. This ongoing demand for new pharmaceutical solutions to combat chronic conditions is, therefore, a significant driver for the growth of the market.
The ADME toxicology testing market is also benefiting from rapid advancements in technology. Modern testing methods, such as in vitro and in silico models, are increasingly being adopted over traditional in vivo methods, offering more accurate, efficient, and cost-effective testing options. These innovative approaches reduce the reliance on animal testing, align with ethical standards, and provide faster results. Moreover, to improve the accuracy of ADME studies, various companies have introduced chips with materials that minimize drug absorption. For instance, in September 2024, Emulate, Inc., a producer of next-generation in vitro organ-chip models, released the new chip-R1, designed to limit medication absorption and improve biological modeling. Chip-R1, built with low-drug-absorbing polymers, expands on the underlying microfluidic architecture of Organ-Chips, giving researchers greater precision in predicting human drug responses. Besides this, one of the primary factors driving the ADME is the significant increase in research and development expenditure in the pharmaceutical sector. As companies invest more in developing new immunotherapies, there is a heightened need for comprehensive ADME toxicology testing to ensure drug safety and efficacy. For instance, in September 2024, Insilico Medicine, a clinical-stage generative artificial intelligence (AI)-driven biotechnology company, collaborated with Inimmune, which will use Chemistry42, Insilico's proprietary generative artificial intelligence (AI) technology, to accelerate the discovery and development of next-generation immunotherapies. Chemistry42 permits the synthesis and design of pharmacological compounds with specific physicochemical features from the start. It also allows for the assessment of multidimensional aspects such as pharmacological efficacy, metabolic stability, synthetic difficulty, ADME qualities, and the selectivity of the produced compounds.
Global ADME Toxicology Testing Market Statistics, By Region
The market research report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share, driven primarily by the extensive pharmaceutical and biotechnology industry in the United States and Canada. The region's robust research and development activities, stringent regulatory requirements, and the presence of major pharmaceutical companies contribute significantly to market growth.
North America ADME Toxicology Testing Market Trends:
The increasing demand for personalized medicine and precision drug development is propelling the adoption of ADME toxicology testing in this region. Technological advancements and collaborations between industry and academic institutions further enhance the market's expansion. For instance, in September 2024, BioIVT, a research partner and biospecimen solutions provider for drug and diagnostic development, announced to host trending topics in ADME & drug development research, a complimentary symposium, at the Mass Bio Hub in Cambridge, Massachusetts, U.S. to discuss novel vitro ADME research methodologies that can be incorporated into preclinical programs to guide lead selection and optimization and improve IVIVC.
Europe ADME Toxicology Testing Market Trends:
There has been a significant surge in the prevalence of chronic diseases such as cardiovascular diseases, diabetes, and cancer across the region. For instance, according to an article published by the European Commission, over 33 million people in the EU have diabetes. Also, according to IDF (International Diabetes Federation) data, the number of diabetics in the EU will increase from 33 million in 2010 to 38 million by 2030. This surge in chronic diseases necessitates the development of new therapeutic agents, which in turn requires comprehensive ADME toxicology testing to ensure drug safety and efficacy, further propelling the market's growth.
Asia Pacific ADME Toxicology Testing Market Trends:
Countries like China and India have seen a surge in pharmaceutical and biotechnology research and development. This uptick necessitates comprehensive ADME toxicology testing to assess the safety and efficacy of new compounds, thereby escalating the market demand.
Latin America ADME Toxicology Testing Market Trends:
The increasing incidence of chronic diseases in Latin America is leading to a higher demand for new therapeutic drugs. For instance, according to an article published by BMC Public Health, approximately 70% of people in Brazil develop a chronic disease by the age of 60. This surge necessitates extensive ADME toxicology testing to ensure the development of safe and effective treatments.
Middle East and Africa ADME Toxicology Testing Market Trends:
Countries such as the United Arab Emirates and South Africa are witnessing significant growth in their pharmaceutical industries. This expansion is leading to increased demand for ADME toxicology testing to ensure drug safety and efficacy.
Top Companies Leading in the ADME Toxicology Testing Industry
Some of the leading ADME toxicology testing market companies include Agilent Technologies Inc., Beckman Coulter Inc. (Danaher Corporation), Bioivt LLC, Bio-Rad Laboratories Inc., Charles River Laboratories International Inc., Cyprotex Plc (Evotec AG), Molecular Discovery Ltd., Perkinelmer Inc., Promega Corporation, and Thermo Fisher Scientific Inc., among many others. For instance, in September 2024, Agilent Technologies Inc. acquired BIOVECTRA, a Canadian contract development and manufacturing organization (CDMO) that focuses on biologics and other compounds for targeted therapies. Also, in October 2024, Thermo Fisher Scientific signed a Memorandum of Understanding (MoU) with the Telangana government to construct a Bioprocess Design Centre (BDC) in Genome Valley, Hyderabad. The Bioprocess Design Centre, spanning 10,000 square feet, will open in early 2025 and serve as a benchmark for accelerating the research and production of new biotherapeutics in India and the Asia-Pacific region.
Global ADME Toxicology Testing Market Segmentation Coverage
- On the basis of the technology, the market has been bifurcated into cell culture, high throughput screening, molecular imaging, OMICS technology, and others, wherein cell culture represented the largest segment. Cell culture-based ADME testing provides valuable insights into drug absorption, metabolism, and toxicity within living cells, making it an essential component of preclinical drug development.
- Based on the product type, the market is categorized into instruments, software solutions, assay systems, reagents, and others, amongst which software solutions accounted for the largest market share. The growing emphasis on data-driven decision-making and the need for efficient data management has propelled the dominance of software solutions in the market.
- On the basis of the method, the market has been divided into in-vivo, in-vitro, in-silica, and others. Among these, in-vivo accounted for the largest market share due to regulatory requirements that often mandate animal testing before human trials, making it a fundamental component of pharmaceutical research and development.
- Based on the application, the market is bifurcated into systemic toxicity, renal toxicity, hepatotoxicity, neurotoxicity, and others, wherein systemic toxicity represents the largest segment. Systemic toxicity testing is vital in pharmaceutical and chemical industries to evaluate the overall safety of drugs and compounds before they are introduced into clinical trials or marketed to the public, further acting as a growth-inducing factor.
| Report Features | Details |
|---|---|
| Market Size in 2024 | USD 10.3 Billion |
| Market Forecast in 2033 | USD 30.2 Billion |
| Market Growth Rate 2025-2033 | 11.32% |
| Units | Billion USD |
| Scope of the Report | Exploration of Historical Trends and Market Outlook, Industry Catalysts and Challenges, Segment-Wise Historical and Future Market Assessment:
|
| Technologies Covered | Cell Culture, High Throughput Screening, Molecular Imaging, OMICS Technology, Others |
| Product Types Covered | Instruments, Software Solutions, Assay Systems, Reagents, Others |
| Methods Covered | In-Vivo, In-Vitro, In-Silica, Others |
| Applications Covered | Systemic Toxicity, Renal Toxicity, Hepatotoxicity, Neurotoxicity, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East and Africa |
| Countries Covered | United States, Canada, Germany, France, United Kingdom, Italy, Spain, Russia, China, Japan, India, South Korea, Australia, Indonesia, Brazil, Mexico |
| Companies Covered | Agilent Technologies Inc., Beckman Coulter Inc. (Danaher Corporation), Bioivt LLC, Bio-Rad Laboratories Inc., Charles River Laboratories International Inc., Cyprotex Plc (Evotec AG), Molecular Discovery Ltd., Perkinelmer Inc., Promega Corporation, Thermo Fisher Scientific Inc., etc. |
| Customization Scope | 10% Free Customization |
| Post-Sale Analyst Support | 10-12 Weeks |
| Delivery Format | PDF and Excel through Email (We can also provide the editable version of the report in PPT/Word format on special request) |
Need more help?
- Speak to our experienced analysts for insights on the current market scenarios.
- Include additional segments and countries to customize the report as per your requirement.
- Gain an unparalleled competitive advantage in your domain by understanding how to utilize the report and positively impacting your operations and revenue.
- For further assistance, please connect with our analysts.





.webp)




.webp)












